Importance of ALDH1A enzymes in determining human testicular retinoic acid concentrations
- PMID: 25502770
- PMCID: PMC4306688
- DOI: 10.1194/jlr.M054718
Importance of ALDH1A enzymes in determining human testicular retinoic acid concentrations
Abstract
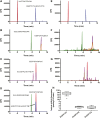
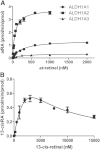
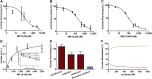
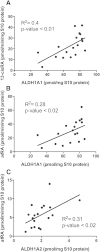
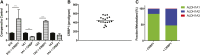
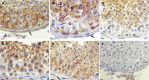
Retinoic acid (RA), the active metabolite of vitamin A, is required for spermatogenesis and many other biological processes. RA formation requires irreversible oxidation of retinal to RA by aldehyde dehydrogenase enzymes of the 1A family (ALDH1A). While ALDH1A1, ALDH1A2, and ALDH1A3 all form RA, the expression pattern and relative contribution of these enzymes to RA formation in the testis is unknown. In this study, novel methods to measure ALDH1A protein levels and intrinsic RA formation were used to accurately predict RA formation velocities in individual human testis samples and an association between RA formation and intratesticular RA concentrations was observed. The distinct localization of ALDH1A in the testis suggests a specific role for each enzyme in controlling RA formation. ALDH1A1 was found in Sertoli cells, while only ALDH1A2 was found in spermatogonia, spermatids, and spermatocytes. In the absence of cellular retinol binding protein (CRBP)1, ALDH1A1 was predicted to be the main contributor to intratesticular RA formation, but when CRBP1 was present, ALDH1A2 was predicted to be equally important in RA formation as ALDH1A1. This study provides a comprehensive novel methodology to evaluate RA homeostasis in human tissues and provides insight to how the individual ALDH1A enzymes mediate RA concentrations in specific cell types.
Keywords: aldehyde dehydrogenase 1A; mass spectrometry; testes.
Copyright © 2015 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Noy N. 2010. Between death and survival: retinoic acid in regulation of apoptosis. Annu. Rev. Nutr. 30: 201–217. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

