A massively parallel pipeline to clone DNA variants and examine molecular phenotypes of human disease mutations
- PMID: 25502805
- PMCID: PMC4263371
- DOI: 10.1371/journal.pgen.1004819
A massively parallel pipeline to clone DNA variants and examine molecular phenotypes of human disease mutations
Abstract
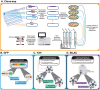
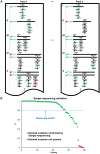
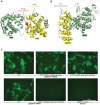
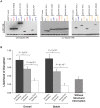
Understanding the functional relevance of DNA variants is essential for all exome and genome sequencing projects. However, current mutagenesis cloning protocols require Sanger sequencing, and thus are prohibitively costly and labor-intensive. We describe a massively-parallel site-directed mutagenesis approach, "Clone-seq", leveraging next-generation sequencing to rapidly and cost-effectively generate a large number of mutant alleles. Using Clone-seq, we further develop a comparative interactome-scanning pipeline integrating high-throughput GFP, yeast two-hybrid (Y2H), and mass spectrometry assays to systematically evaluate the functional impact of mutations on protein stability and interactions. We use this pipeline to show that disease mutations on protein-protein interaction interfaces are significantly more likely than those away from interfaces to disrupt corresponding interactions. We also find that mutation pairs with similar molecular phenotypes in terms of both protein stability and interactions are significantly more likely to cause the same disease than those with different molecular phenotypes, validating the in vivo biological relevance of our high-throughput GFP and Y2H assays, and indicating that both assays can be used to determine candidate disease mutations in the future. The general scheme of our experimental pipeline can be readily expanded to other types of interactome-mapping methods to comprehensively evaluate the functional relevance of all DNA variants, including those in non-coding regions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

