Use of a Guinea pig-specific transcriptome array for evaluation of protective immunity against genital chlamydial infection following intranasal vaccination in Guinea pigs
- PMID: 25502875
- PMCID: PMC4263467
- DOI: 10.1371/journal.pone.0114261
Use of a Guinea pig-specific transcriptome array for evaluation of protective immunity against genital chlamydial infection following intranasal vaccination in Guinea pigs
Abstract
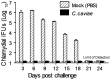
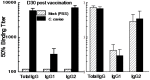
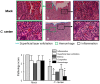
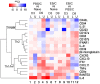
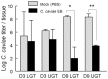
Guinea pigs have been used as a second animal model to validate putative anti-chlamydial vaccine candidates tested in mice. However, the lack of guinea pig-specific reagents has limited the utility of this animal model in Chlamydia sp. vaccine studies. Using a novel guinea pig-specific transcriptome array, we determined correlates of protection in guinea pigs vaccinated with Chlamydia caviae (C. caviae) via the intranasal route, previously reported by us and others to provide robust antigen specific immunity against subsequent intravaginal challenge. C. caviae vaccinated guinea pigs resolved genital infection by day 3 post challenge. In contrast, mock vaccinated animals continued to shed viable Chlamydia up to day 18 post challenge. Importantly, at day 80 post challenge, vaccinated guinea pigs experienced significantly reduced genital pathology - a sequelae of genital chlamydial infections, in comparison to mock vaccinated guinea pigs. Sera from vaccinated guinea pigs displayed antigen specific IgG responses and increased IgG1 and IgG2 titers capable of neutralizing GPIC in vitro. Th1-cellular/inflammatory immune genes and Th2-humoral associated genes were also found to be elevated in vaccinated guinea pigs at day 3 post-challenge and correlated with early clearance of the bacterium. Overall, this study provides the first evidence of guinea pig-specific genes involved in anti-chlamydial vaccination and illustrates the enhancement of the utility of this animal model in chlamydial pathogenesis.
Conflict of interest statement
Figures

References
-
- Beagley KW, Timms P (2000) Chlamydia trachomatis infection: incidence, health costs and prospects for vaccine development. J Reprod Immunol 48:47–68. - PubMed
-
- Waight MT, Rahman MM, Soto P, Tran T (2013) Sexually transmitted diseases during pregnancy in Louisiana, 2007–2009: high-risk populations and adverse newborn outcomes. Journal of the Louisiana State Medical Society 165:219–226. - PubMed
-
- Folger AT (2013) Maternal Chlamydia trachomatis Infections and Preterm Birth: The Impact of Early Detection and Eradication During Pregnancy. Maternal and Child Health Journal. - PubMed
-
- Gaydos CA, Barnes M, Jett-Goheen M, Quinn N, Whittle P, et al. (2013) Characteristics and predictors of women who obtain rescreening for sexually transmitted infections using the www.iwantthekit.org screening programme., International Journal of STD and AIDS 24:736–744. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

