Inhibitory/suppressive oligodeoxynucleotide nanocapsules as simple oral delivery devices for preventing atopic dermatitis in mice
- PMID: 25502904
- PMCID: PMC4445625
- DOI: 10.1038/mt.2014.239
Inhibitory/suppressive oligodeoxynucleotide nanocapsules as simple oral delivery devices for preventing atopic dermatitis in mice
Abstract
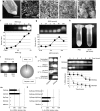
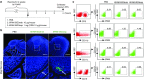
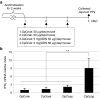
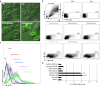
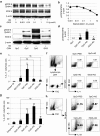
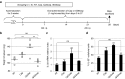
Here, we report a simple and low-cost oral oligodeoxynucleotide (ODN) delivery system targeted to the gut Peyer's patches (PPs). This system requires only Dulbecco's modified eagle's medium, calcium chloride, ODNs, and basic laboratory equipment. ODN nanocapsules (ODNcaps) were directly delivered to the PPs through oral administration and were taken up by macrophages in the PPs, where they induced an immune response. Long-term continuous oral dosing with inhibitory/suppressive ODNcaps (iODNcaps, "iSG3caps" in this study) was evaluated using an atopic dermatitis mouse model to visually monitor disease course. Administration of iSG3caps improved skin lesions and decreased epidermal thickness. Underlying this effect is the ability of iSG3 to bind to and prevent phosphorylation of signal transducer and activator of transcription 6, thereby blocking the interleukin-4 signaling cascade mediated by binding of allergens to type 2 helper T cells. The results of our iSG3cap oral delivery experiments suggest that iSG3 may be useful for treating allergic diseases.
Figures


Comment in
-
Therapeutic implications of orally delivered immunomodulatory oligonucleotides.Mol Ther. 2015 Feb;23(2):222-3. doi: 10.1038/mt.2014.251. Mol Ther. 2015. PMID: 25633172 Free PMC article. No abstract available.
Similar articles
-
In vivo transfection of a cis element 'decoy' against signal transducers and activators of transcription 6 (STAT6)-binding site ameliorates IgE-mediated late-phase reaction in an atopic dermatitis mouse model.Gene Ther. 2004 Dec;11(24):1753-62. doi: 10.1038/sj.gt.3302341. Gene Ther. 2004. PMID: 15306842
-
Hyperproduction of IFN-gamma by CpG oligodeoxynucleotide-induced exacerbation of atopic dermatitis-like skin lesion in some NC/Nga mice.J Invest Dermatol. 2005 Dec;125(6):1156-62. doi: 10.1111/j.0022-202X.2005.23928.x. J Invest Dermatol. 2005. PMID: 16354185
-
Effect of the Lactococcus Lactis 11/19-B1 Strain on Atopic Dermatitis in a Clinical Test and Mouse Model.Nutrients. 2020 Mar 14;12(3):763. doi: 10.3390/nu12030763. Nutrients. 2020. PMID: 32183266 Free PMC article. Clinical Trial.
-
Therapeutic implications of orally delivered immunomodulatory oligonucleotides.Mol Ther. 2015 Feb;23(2):222-3. doi: 10.1038/mt.2014.251. Mol Ther. 2015. PMID: 25633172 Free PMC article. No abstract available.
-
Particle uptake by Peyer's patches: a pathway for drug and vaccine delivery.Expert Opin Drug Deliv. 2004 Nov;1(1):141-63. doi: 10.1517/17425247.1.1.141. Expert Opin Drug Deliv. 2004. PMID: 16296726 Review.
Cited by
-
Adjuvanted Immunotherapy Approaches for Peanut Allergy.Front Immunol. 2018 Sep 25;9:2156. doi: 10.3389/fimmu.2018.02156. eCollection 2018. Front Immunol. 2018. PMID: 30319619 Free PMC article. Review.
-
Oral delivery of Lactococcus lactis that secretes bioactive heme oxygenase-1 alleviates development of acute colitis in mice.Microb Cell Fact. 2015 Nov 25;14:189. doi: 10.1186/s12934-015-0378-2. Microb Cell Fact. 2015. PMID: 26608030 Free PMC article.
-
Synergistic oligodeoxynucleotide strongly promotes CpG-induced interleukin-6 production.BMC Immunol. 2017 Oct 4;18(1):44. doi: 10.1186/s12865-017-0227-7. BMC Immunol. 2017. PMID: 28978323 Free PMC article.
-
Cooperation of Oligodeoxynucleotides and Synthetic Molecules as Enhanced Immune Modulators.Front Nutr. 2019 Aug 27;6:140. doi: 10.3389/fnut.2019.00140. eCollection 2019. Front Nutr. 2019. PMID: 31508424 Free PMC article. Review.
-
Structure, mechanism and therapeutic utility of immunosuppressive oligonucleotides.Pharmacol Res. 2016 Mar;105:216-25. doi: 10.1016/j.phrs.2015.11.010. Epub 2016 Jan 15. Pharmacol Res. 2016. PMID: 26779666 Free PMC article. Review.
References
-
- Karbach J, Neumann A, Wahle C, Brand K, Gnjatic S, Jäger E. Therapeutic administration of a synthetic CpG oligodeoxynucleotide triggers formation of anti-CpG antibodies. Cancer Res. 2012;72:4304–4310. - PubMed
-
- Klinman DM. Immunotherapeutic uses of CpG oligodeoxynucleotides. Nat Rev Immunol. 2004;4:249–258. - PubMed
-
- Pico de Coana Y, Carnes J, Gallego MT, Alonso C, Parody N. Modulation of the humoral response to Dermatophagoides pteronyssinus allergens in BALB/c mice by extract modification and adjuvant use. Int Arch Allergy Immunol. 2012;157:331–338. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

