Catalytic enantioselective synthesis of quaternary carbon stereocentres
- PMID: 25503231
- PMCID: PMC4697831
- DOI: 10.1038/nature14007
Catalytic enantioselective synthesis of quaternary carbon stereocentres
Abstract
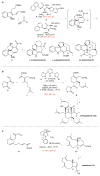
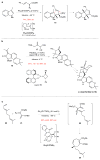
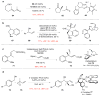
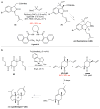
Quaternary carbon stereocentres-carbon atoms to which four distinct carbon substituents are attached-are common features of molecules found in nature. However, before recent advances in chemical catalysis, there were few methods of constructing single stereoisomers of this important structural motif. Here we discuss the many catalytic enantioselective reactions developed during the past decade for the synthesis of single stereoisomers of such organic molecules. This progress now makes it possible to incorporate quaternary stereocentres selectively in many organic molecules that are useful in medicine, agriculture and potentially other areas such as flavouring, fragrances and materials.
Figures





References
-
- Bartholow M. Top 200 drugs of 2011. Pharmacy Times. 2012;(July):48–51.
-
- Ding HX, Liu KK-C, Sakya SM, Flick AC, O’Donnell CJ. Synthetic approaches to the 2011 new drugs. Bioorg Med Chem. 2013;21:2795–2825. and previous reviews in this series. - PubMed
-
- Christoffers J, Baro A, editors. Quaternary Stereocenters–Challenges and Solutions for Organic Synthesis. Wiley–VCH; Weinheim: 2005.
-
- Hong AY, Stoltz BM. The construction of all-carbon quaternary stereocenters by use of Pd-catalyzed asymmetric allylic alkylation reactions in total synthesis. Eur J Org Chem. 2013:2745–2759. and previous reviews of enantioselective synthesis of quaternary stereocenters cited in footnote 2 therein. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

