The carboxy-terminus of p63 links cell cycle control and the proliferative potential of epidermal progenitor cells
- PMID: 25503409
- PMCID: PMC4302837
- DOI: 10.1242/dev.118307
The carboxy-terminus of p63 links cell cycle control and the proliferative potential of epidermal progenitor cells
Abstract
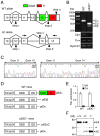
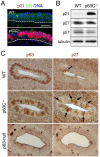
The transcription factor p63 (Trp63) plays a key role in homeostasis and regeneration of the skin. The p63 gene is transcribed from dual promoters, generating TAp63 isoforms with growth suppressive functions and dominant-negative ΔNp63 isoforms with opposing properties. p63 also encodes multiple carboxy (C)-terminal variants. Although mutations of C-terminal variants have been linked to the pathogenesis of p63-associated ectodermal disorders, the physiological role of the p63 C-terminus is poorly understood. We report here that deletion of the p63 C-terminus in mice leads to ectodermal malformation and hypoplasia, accompanied by a reduced proliferative capacity of epidermal progenitor cells. Notably, unlike the p63-null condition, we find that p63 C-terminus deficiency promotes expression of the cyclin-dependent kinase inhibitor p21(Waf1/Cip1) (Cdkn1a), a factor associated with reduced proliferative capacity of both hematopoietic and neuronal stem cells. These data suggest that the p63 C-terminus plays a key role in the cell cycle progression required to maintain the proliferative potential of stem cells of many different lineages. Mechanistically, we show that loss of Cα, the predominant C-terminal p63 variant in epithelia, promotes the transcriptional activity of TAp63 and also impairs the dominant-negative activity of ΔNp63, thereby controlling p21(Waf1/Cip1) expression. We propose that the p63 C-terminus links cell cycle control and the proliferative potential of epidermal progenitor cells via mechanisms that equilibrate TAp63 and ΔNp63 isoform function.
Keywords: Cell cycle; Epithelia; Mouse; Proliferation; Stem cells; p21; p63.
© 2015. Published by The Company of Biologists Ltd.
Figures




Similar articles
-
Unique domain functions of p63 isotypes that differentially regulate distinct aspects of epidermal homeostasis.Carcinogenesis. 2006 Jan;27(1):53-63. doi: 10.1093/carcin/bgi200. Epub 2005 Aug 4. Carcinogenesis. 2006. PMID: 16081516
-
Differential expression of p63 isoforms in normal tissues and neoplastic cells.J Pathol. 2002 Dec;198(4):417-27. doi: 10.1002/path.1231. J Pathol. 2002. PMID: 12434410
-
ΔNp63 knockout mice reveal its indispensable role as a master regulator of epithelial development and differentiation.Development. 2012 Feb;139(4):772-82. doi: 10.1242/dev.071191. Development. 2012. PMID: 22274697 Free PMC article.
-
TAp63 and DeltaNp63 in cancer and epidermal development.Cell Cycle. 2007 Feb 1;6(3):274-85. doi: 10.4161/cc.6.3.3797. Epub 2007 Feb 3. Cell Cycle. 2007. PMID: 17264681 Review.
-
Epidermal differentiation: transgenic/knockout mouse models reveal genes involved in stem cell fate decisions and commitment to differentiation.J Investig Dermatol Symp Proc. 2002 Dec;7(1):41-5. doi: 10.1046/j.1523-1747.2002.19639.x. J Investig Dermatol Symp Proc. 2002. PMID: 12518791 Review.
Cited by
-
Pathway Regulation of p63, a Director of Epithelial Cell Fate.Front Endocrinol (Lausanne). 2015 Apr 28;6:51. doi: 10.3389/fendo.2015.00051. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25972840 Free PMC article. Review.
-
p73 Alternative Splicing: Exploring a Biological Role for the C-Terminal Isoforms.J Mol Biol. 2018 Jun 22;430(13):1829-1838. doi: 10.1016/j.jmb.2018.04.034. Epub 2018 May 4. J Mol Biol. 2018. PMID: 29733853 Free PMC article. Review.
-
Deletion of p63 exon 13 in mice reveals C-terminal isoform-specific functions in epithelial development.Proc Natl Acad Sci U S A. 2025 Jul 22;122(29):e2503866122. doi: 10.1073/pnas.2503866122. Epub 2025 Jul 17. Proc Natl Acad Sci U S A. 2025. PMID: 40674423 Free PMC article.
-
Wnt/β-catenin signaling controls mouse eyelid growth by mediating epithelial-mesenchymal interactions.Ocul Surf. 2023 Jul;29:486-494. doi: 10.1016/j.jtos.2023.07.007. Epub 2023 Jul 13. Ocul Surf. 2023. PMID: 37453535 Free PMC article.
-
SMG1 and CDK12 Link ΔNp63α Phosphorylation to RNA Surveillance in Keratinocytes.J Proteome Res. 2021 Dec 3;20(12):5347-5358. doi: 10.1021/acs.jproteome.1c00427. Epub 2021 Nov 11. J Proteome Res. 2021. PMID: 34761935 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

