α-Tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration
- PMID: 25503560
- PMCID: PMC4350791
- DOI: 10.1158/0008-5472.CAN-13-3563
α-Tubulin acetylation elevated in metastatic and basal-like breast cancer cells promotes microtentacle formation, adhesion, and invasive migration
Abstract
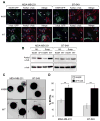
Metastatic cases of breast cancer pose the primary challenge in clinical management of this disease, demanding the identification of effective therapeutic strategies that remain wanting. In this study, we report that elevated levels of α-tubulin acetylation are a sufficient cause of metastatic potential in breast cancer. In suspended cell culture conditions, metastatic breast cancer cells exhibited high α-tubulin acetylation levels that extended along microtentacle (McTN) protrusions. Mutation of the acetylation site on α-tubulin and enzymatic modulation of this posttranslational modification exerted a significant impact on McTN frequency and the reattachment of suspended tumor cells. Reducing α-tubulin acetylation significantly inhibited migration but did not affect proliferation. In an analysis of more than 140 matched primary and metastatic tumors from patients, we found that acetylation was maintained and in many cases increased in lymph node metastases compared with primary tumors. Proteomic analysis of an independent cohort of more than 390 patient specimens further documented the relationship between increased α-tubulin acetylation and the aggressive behaviors of basal-like breast cancers, with a trend toward increased risk of disease progression and death in patients with high-intensity α-tubulin acetylation in primary tumors. Taken together, our results identify a tight correlation between acetylated α-tubulin levels and aggressive metastatic behavior in breast cancer, with potential implications for the definition of a simple prognostic biomarker in patients with breast cancer.
©2014 American Association for Cancer Research.
Conflict of interest statement
Figures






Similar articles
-
Metastatic breast tumors express increased tau, which promotes microtentacle formation and the reattachment of detached breast tumor cells.Oncogene. 2010 Jun 3;29(22):3217-27. doi: 10.1038/onc.2010.68. Epub 2010 Mar 15. Oncogene. 2010. PMID: 20228842 Free PMC article.
-
Hydrogen Peroxide Induces α-Tubulin Detyrosination and Acetylation and Impacts Breast Cancer Metastatic Phenotypes.Cells. 2023 Apr 27;12(9):1266. doi: 10.3390/cells12091266. Cells. 2023. PMID: 37174666 Free PMC article.
-
The role of Runx2 in facilitating autophagy in metastatic breast cancer cells.J Cell Physiol. 2018 Jan;233(1):559-571. doi: 10.1002/jcp.25916. Epub 2017 May 19. J Cell Physiol. 2018. PMID: 28345763
-
Local anesthetics inhibit kinesin motility and microtentacle protrusions in human epithelial and breast tumor cells.Breast Cancer Res Treat. 2011 Oct;129(3):691-701. doi: 10.1007/s10549-010-1239-7. Epub 2010 Nov 11. Breast Cancer Res Treat. 2011. PMID: 21069453 Free PMC article.
-
The oncoprotein HBXIP promotes migration of breast cancer cells via GCN5-mediated microtubule acetylation.Biochem Biophys Res Commun. 2015 Mar 13;458(3):720-725. doi: 10.1016/j.bbrc.2015.02.036. Epub 2015 Feb 14. Biochem Biophys Res Commun. 2015. PMID: 25686500
Cited by
-
Tubulin acetylation enhances lung cancer resistance to paclitaxel-induced cell death through Mcl-1 stabilization.Cell Death Discov. 2021 Apr 6;7(1):67. doi: 10.1038/s41420-021-00453-9. Cell Death Discov. 2021. PMID: 33824297 Free PMC article.
-
Lysine acetyltransferases and lysine deacetylases as targets for cardiovascular disease.Nat Rev Cardiol. 2020 Feb;17(2):96-115. doi: 10.1038/s41569-019-0235-9. Epub 2019 Jul 26. Nat Rev Cardiol. 2020. PMID: 31350538 Review.
-
Microtubule Hyperacetylation Enhances KL1-Dependent Micronucleation under a Tau Deficiency in Mammary Epithelial Cells.Int J Mol Sci. 2018 Aug 23;19(9):2488. doi: 10.3390/ijms19092488. Int J Mol Sci. 2018. PMID: 30142893 Free PMC article.
-
Emerging Role of Histone Acetyltransferase in Stem Cells and Cancer.Stem Cells Int. 2018 Dec 16;2018:8908751. doi: 10.1155/2018/8908751. eCollection 2018. Stem Cells Int. 2018. PMID: 30651738 Free PMC article. Review.
-
Post-translational modifications of tubulin: their role in cancers and the regulation of signaling molecules.Cancer Gene Ther. 2023 Apr;30(4):521-528. doi: 10.1038/s41417-021-00396-4. Epub 2021 Oct 20. Cancer Gene Ther. 2023. PMID: 34671113 Review.
References
-
- ACS. American Cancer Society Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2013.
-
- Eckhardt BL, Francis PA, Parker BS, Anderson RL. Strategies for the discovery and development of therapies for metastatic breast cancer. Nat Rev Drug Discov. 2012;11:479–97. - PubMed
-
- Hall A. The cytoskeleton and cancer. Cancer Metastasis Rev. 2009;28:5–14. - PubMed
-
- Korb T, Schluter K, Enns A, Spiegel HU, Senninger N, Nicolson GL, et al. Integrity of actin fibers and microtubules influences metastatic tumor cell adhesion. Exp Cell Res. 2004;299:236–47. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

