Biomodulatory Treatment of Patients with Castration-Resistant Prostate Cancer: A Phase II Study of Imatinib with Pioglitazone, Etoricoxib, Dexamethasone and Low-Dose Treosulfan
- PMID: 25503648
- PMCID: PMC4449347
- DOI: 10.1007/s12307-014-0161-7
Biomodulatory Treatment of Patients with Castration-Resistant Prostate Cancer: A Phase II Study of Imatinib with Pioglitazone, Etoricoxib, Dexamethasone and Low-Dose Treosulfan
Erratum in
-
Erratum to: Biomodulatory Treatment of Patients with Castration-Resistant Prostate Cancer: A Phase II Study of Imatinib with Pioglitazone, Etoricoxib, Dexamethasone and Low-Dose Treosulfan.Cancer Microenviron. 2015 Apr;8(1):43-4. doi: 10.1007/s12307-015-0165-y. Cancer Microenviron. 2015. PMID: 25651886 Free PMC article. No abstract available.
Abstract
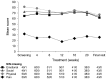
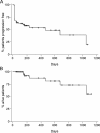
Therapeutic options for patients with castration-resistant prostate cancer (CRPC) remain limited. In a multicenter, Phase II study, 65 patients with histologically confirmed CRPC received a biomodulatory regimen during the six-month core study. Treatment comprised daily doses of imatinib mesylate, pioglitazone, etoricoxib, treosulfan and dexamethasone. The primary endpoint was prostate-specific antigen (PSA) response. Responders could enter an extension phase until disease progression or intolerable toxicity occurred. Mean PSA was 45.3 ng/mL at baseline, and 77 % of patients had a PSA doubling time <3 months. Of the 61 evaluable patients, 37 patients (60.6 %) responded or had stable disease and 23 of them (37.7 % of 61 patients) were PSA responders. Among the 23 responders mean PSA decreased from 278.9 ± 784.1 ng/mL at baseline to 8.8 ± 11.6 ng/mL at the final visit (week 24). The progression-free survival (PFS) was 467 days in the ITT population. Of the 947 adverse events, 57.6 % were suspected to be drug-related, 13.8 % led to dose adjustment or permanent discontinuation and 40.2 % required concomitant medication. This novel combination approach led to an impressive PSA response rate of 37.7 % in CRPC patients. The good PSA response and PFS rate combined with the manageable toxicity profile suggest an alternative treatment option.
Conflict of interest statement
The study was funded by Novartis Pharma GmbH, Nuernberg, Germany. Amelie Ruebel, Katrin Birkholz, Katja Schmidt and Monika Baier are employees of Novartis Pharma GmbH and contributed to the set-up, conduct, and analysis of the study. The other authors declare that they have no conflict of interest.
Figures


Similar articles
-
Modular therapy approach in metastatic castration-refractory prostate cancer.World J Urol. 2010 Dec;28(6):745-50. doi: 10.1007/s00345-010-0567-x. Epub 2010 May 19. World J Urol. 2010. PMID: 20490506 Clinical Trial.
-
Erratum to: Biomodulatory Treatment of Patients with Castration-Resistant Prostate Cancer: A Phase II Study of Imatinib with Pioglitazone, Etoricoxib, Dexamethasone and Low-Dose Treosulfan.Cancer Microenviron. 2015 Apr;8(1):43-4. doi: 10.1007/s12307-015-0165-y. Cancer Microenviron. 2015. PMID: 25651886 Free PMC article. No abstract available.
-
Prostate-specific antigen response to deferred combined androgen blockade therapy using bicalutamide predicts survival after subsequent oestrogen and docetaxel therapies in patients with castration-resistant prostate cancer.BJU Int. 2012 Oct;110(8):1149-55. doi: 10.1111/j.1464-410X.2012.10959.x. Epub 2012 Feb 28. BJU Int. 2012. PMID: 22369348
-
Phase II study of single-agent orteronel (TAK-700) in patients with nonmetastatic castration-resistant prostate cancer and rising prostate-specific antigen.Clin Cancer Res. 2014 Aug 15;20(16):4218-27. doi: 10.1158/1078-0432.CCR-14-0356. Epub 2014 Jun 25. Clin Cancer Res. 2014. PMID: 24965748 Clinical Trial.
-
A Phase 2 Randomized Controlled Trial of Personalized Peptide Vaccine Immunotherapy with Low-dose Dexamethasone Versus Dexamethasone Alone in Chemotherapy-naive Castration-resistant Prostate Cancer.Eur Urol. 2016 Jul;70(1):35-41. doi: 10.1016/j.eururo.2015.12.050. Epub 2016 Jan 15. Eur Urol. 2016. PMID: 26782346 Clinical Trial.
Cited by
-
Peroxisome Proliferator-Activated Receptors (PPAR)γ Agonists as Master Modulators of Tumor Tissue.Int J Mol Sci. 2018 Nov 9;19(11):3540. doi: 10.3390/ijms19113540. Int J Mol Sci. 2018. PMID: 30424016 Free PMC article. Review.
-
Peroxisome proliferator-activated receptorα/γ agonist pioglitazone for rescuing relapsed or refractory neoplasias by unlocking phenotypic plasticity.Front Oncol. 2024 Jan 11;13:1289222. doi: 10.3389/fonc.2023.1289222. eCollection 2023. Front Oncol. 2024. PMID: 38273846 Free PMC article. Review.
-
Drug Repurposing by Tumor Tissue Editing.Front Oncol. 2022 Jun 24;12:900985. doi: 10.3389/fonc.2022.900985. eCollection 2022. Front Oncol. 2022. PMID: 35814409 Free PMC article. Review.
-
Clinical Efficacy of a Novel Therapeutic Principle, Anakoinosis.Front Pharmacol. 2018 Nov 28;9:1357. doi: 10.3389/fphar.2018.01357. eCollection 2018. Front Pharmacol. 2018. PMID: 30546308 Free PMC article. Review.
-
Etoricoxib-Cannabidiol Combo: Potential Role in Glioblastoma Treatment and Development of PLGA-Based Nanoparticles.Pharmaceutics. 2023 Aug 9;15(8):2104. doi: 10.3390/pharmaceutics15082104. Pharmaceutics. 2023. PMID: 37631318 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

