Bilirubin modulates acetylcholine receptors in rat superior cervical ganglionic neurons in a bidirectional manner
- PMID: 25503810
- PMCID: PMC4265787
- DOI: 10.1038/srep07475
Bilirubin modulates acetylcholine receptors in rat superior cervical ganglionic neurons in a bidirectional manner
Abstract
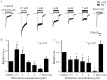
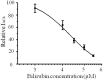
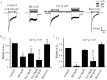
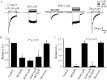
Autonomic dysfunction as a partial contributing factor to cardiovascular instability in jaundiced patients is often associated with increased serum bilirubin levels. Whether increased serum bilirubin levels could directly inhibit sympathetic ganglion transmission by blocking neuronal nicotinic acetylcholine receptors (nAChRs) remains to be elucidated. Conventional patch-clamp recordings were used to study the effect of bilirubin on nAChRs currents from enzymatically dissociated rat superior cervical ganglia (SCG) neurons. The results showed that low concnetrations (0.5 and 2 μM) of bilirubin enhanced the peak ACh-evoked currents, while high concentrations (3 to 5.5 µM) of bilirubin suppressed the currents with an IC50 of 4 ± 0.5 μM. In addition, bilirubin decreased the extent of desensitization of nAChRs in a concentration-dependent manner. This inhibitory effect of bilirubin on nAChRs channel currents was non-competitive and voltage independent. Bilirubin partly improved the inhibitory effect of forskolin on ACh-induced currents without affecting the action of H-89. These data suggest that the dual effects of enhancement and suppression of bilirubin on nAChR function may be ascribed to the action mechanism of positive allosteric modulation and direct blockade. Thus, suppression of sympathetic ganglionic transmission through postganglionic nAChRs inhibition may partially contribute to the adverse cardiovascular effects in jaundiced patients.
Figures
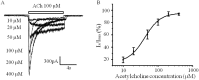

 ) or absence (
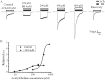
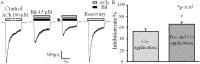
) or absence ( ) of 4.5 μM bilirubin. (B) There was no voltage dependency regarding the effect of 4.5 μM bilirubin on peak nAChRs currents. Each response was normalized to that without bilirubin at each membrane potential from −70 mV to −20 mV. All data points are expressed as means ± SEMs (n = 5).
) of 4.5 μM bilirubin. (B) There was no voltage dependency regarding the effect of 4.5 μM bilirubin on peak nAChRs currents. Each response was normalized to that without bilirubin at each membrane potential from −70 mV to −20 mV. All data points are expressed as means ± SEMs (n = 5).
Similar articles
-
Competitive inhibition of the nondepolarizing muscle relaxant rocuronium on nicotinic acetylcholine receptor channels in the rat superior cervical ganglia.J Cardiovasc Pharmacol. 2014 May;63(5):428-33. doi: 10.1097/FJC.0000000000000063. J Cardiovasc Pharmacol. 2014. PMID: 24803316
-
Alpha2-adrenoceptor-independent inhibition of acetylcholine receptor channel and sodium channel by dexmedetomidine in rat superior cervical ganglion neurons.Neuroscience. 2015 Mar 19;289:9-18. doi: 10.1016/j.neuroscience.2014.12.045. Epub 2015 Jan 9. Neuroscience. 2015. PMID: 25583636
-
Modulation of N-type calcium currents by presynaptic imidazoline receptor activation in rat superior cervical ganglion neurons.Exp Physiol. 2010 Oct;95(10):982-93. doi: 10.1113/expphysiol.2010.053355. Epub 2010 Aug 9. Exp Physiol. 2010. PMID: 20696781
-
Lidocaine effects on acetylcholine-elicited currents from mouse superior cervical ganglion neurons.Neurosci Res. 2013 Mar;75(3):198-203. doi: 10.1016/j.neures.2013.01.005. Epub 2013 Feb 6. Neurosci Res. 2013. PMID: 23395628
-
Molecular mechanisms of open-channel blockade in nicotinic acetylcholine receptors of autonomic ganglia neurons.Can J Physiol Pharmacol. 1992;70 Suppl:S78-85. doi: 10.1139/y92-247. Can J Physiol Pharmacol. 1992. PMID: 1284232 Review.
Cited by
-
High indirect bilirubin levels as an independent predictor of postoperative myasthenic crisis: a single-center, retrospective study.Front Neurol. 2024 Jan 12;14:1336823. doi: 10.3389/fneur.2023.1336823. eCollection 2023. Front Neurol. 2024. PMID: 38283685 Free PMC article.
-
Acute bilirubin ditaurate exposure attenuates ex vivo platelet reactive oxygen species production, granule exocytosis and activation.Redox Biol. 2019 Sep;26:101250. doi: 10.1016/j.redox.2019.101250. Epub 2019 Jun 12. Redox Biol. 2019. PMID: 31226648 Free PMC article.
-
Influence of Nicotine from Diverse Delivery Tools on the Autonomic Nervous and Hormonal Systems.Biomedicines. 2022 Jan 6;10(1):121. doi: 10.3390/biomedicines10010121. Biomedicines. 2022. PMID: 35052800 Free PMC article.
-
Bilirubin potentiates etomidate-induced sedation by enhancing GABA-induced currents after bile duct ligation.BMC Pharmacol Toxicol. 2023 Sep 23;24(1):46. doi: 10.1186/s40360-023-00675-w. BMC Pharmacol Toxicol. 2023. PMID: 37740245 Free PMC article.
References
-
- Yang L. Q. et al. A clinical prospective comparison of anesthetics sensitivity and hemodynamic effect among patients with or without obstructive jaundice. Acta Anaesthesiol Scand 54, 871–877 (2010). - PubMed
-
- Bansal V. & Schuchert V. D. Jaundice in the intensive care unit. Surg Clin North Am 86, 1495–1502 (2006). - PubMed
-
- Wang L. & Yu W. F. Obstructive jaundice and perioperative management. Acta Anaesthesiol Taiwan 52, 22–29 (2014). - PubMed
-
- Green J. & Better O. S. Systemic hypotension and renal failure in obstructive jaundice-mechanistic and therapeutic aspects. J Am Soc Nephrol 5, 1853–1871 (1995). - PubMed
-
- Sitges-Serra A. et al. Body water compartments in patients with obstructive jaundice. Br J Surg 79, 553–556 (1992). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

