Volume-sensitive chloride channels are involved in cisplatin treatment of osteosarcoma
- PMID: 25503821
- PMCID: PMC4337627
- DOI: 10.3892/mmr.2014.3068
Volume-sensitive chloride channels are involved in cisplatin treatment of osteosarcoma
Abstract
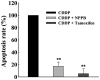
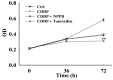
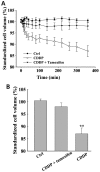
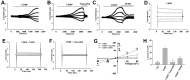
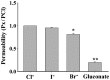
Chemotherapy is the most common therapeutic strategy used to treat osteosarcoma. The present study aimed to investigate the effects of functionally activated chloride channels on cisplatin‑induced apoptosis of MG‑63 human osteosarcoma cells. An MTT assay and flow cytometry were used to detect proliferation and apoptosis of the cells, respectively. Live cell imaging was used to detect volume changes in response to treatment with cisplatin and/or chloride channel blockers. The effects of these treatments on chloride currents were also assayed using the patch‑clamp technique. The results of the present study indicate that chloride channel blockers may suppress cisplatin‑induced apoptosis. The MG‑63 cells cultured with cisplatin demonstrated an apoptotic volume decrease, as well as suppression of cell proliferation; which were reversed by co‑treatment with chloride channel blockers. These results suggest that cisplatin may activate chloride channels, and that channel activation is an early signal in the pathways that lead to cisplatin‑induced apoptosis and inhibition of proliferation in MG‑63 cells. In conclusion, these results indicate that chloride channels have an important role in cisplatin treatment of osteosarcoma.
Figures
Similar articles
-
Enhancement effect of adenovirus-mediated antisense c-myc and caffeine on the cytotoxicity of cisplatin in osteosarcoma cell lines.Chemotherapy. 2009;55(6):433-40. doi: 10.1159/000265527. Epub 2009 Dec 9. Chemotherapy. 2009. PMID: 19996588
-
Impaired activity of volume-sensitive Cl- channel is involved in cisplatin resistance of cancer cells.J Cell Physiol. 2007 May;211(2):513-21. doi: 10.1002/jcp.20961. J Cell Physiol. 2007. PMID: 17186499
-
[Effect of C23 down-regulation on proliferation inhibition and apoptosis induced by cisplatin in human osteosarcoma SaOS-2 cells].Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012 Jan;37(1):32-7. doi: 10.3969/j.issn.1672-7347.2012.01.006. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2012. PMID: 22349377 Chinese.
-
Volume-sensitive Cl(-) channel as a regulator of acquired cisplatin resistance.Anticancer Res. 2008 Jan-Feb;28(1A):75-83. Anticancer Res. 2008. PMID: 18383827 Review.
-
CLC-3 channels in cancer (review).Oncol Rep. 2015 Feb;33(2):507-14. doi: 10.3892/or.2014.3615. Epub 2014 Nov 21. Oncol Rep. 2015. PMID: 25421907 Review.
Cited by
-
Effectiveness of multi-drug regimen chemotherapy treatment in osteosarcoma patients: a network meta-analysis of randomized controlled trials.J Orthop Surg Res. 2017 Mar 29;12(1):52. doi: 10.1186/s13018-017-0544-9. J Orthop Surg Res. 2017. PMID: 28356114 Free PMC article.
-
The Role of Chloride Channels in the Multidrug Resistance.Membranes (Basel). 2021 Dec 28;12(1):38. doi: 10.3390/membranes12010038. Membranes (Basel). 2021. PMID: 35054564 Free PMC article. Review.
-
A correlation analysis between tumor imaging changes and p-AKT and HSP70 expression in tumor cells after osteosarcoma chemotherapy.Oncol Lett. 2017 Dec;14(6):6749-6753. doi: 10.3892/ol.2017.7005. Epub 2017 Sep 20. Oncol Lett. 2017. PMID: 29151914 Free PMC article.
-
Low pH Attenuates Apoptosis by Suppressing the Volume-Sensitive Outwardly Rectifying (VSOR) Chloride Current in Chondrocytes.Front Cell Dev Biol. 2022 Feb 2;9:804105. doi: 10.3389/fcell.2021.804105. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35186954 Free PMC article.
-
Physiological Functions of the Volume-Regulated Anion Channel VRAC/LRRC8 and the Proton-Activated Chloride Channel ASOR/TMEM206.Handb Exp Pharmacol. 2024;283:181-218. doi: 10.1007/164_2023_673. Handb Exp Pharmacol. 2024. PMID: 37468723
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

