Hemodynamic forces regulate developmental patterning of atrial conduction
- PMID: 25503944
- PMCID: PMC4264946
- DOI: 10.1371/journal.pone.0115207
Hemodynamic forces regulate developmental patterning of atrial conduction
Abstract
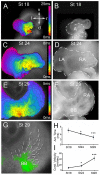
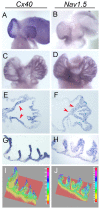
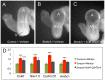
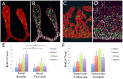
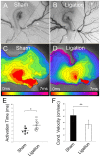
Anomalous action potential conduction through the atrial chambers of the heart can lead to severe cardiac arrhythmia. To date, however, little is known regarding the mechanisms that pattern proper atrial conduction during development. Here we demonstrate that atrial muscle functionally diversifies into at least two heterogeneous subtypes, thin-walled myocardium and rapidly conducting muscle bundles, during a developmental window just following cardiac looping. During this process, atrial muscle bundles become enriched for the fast conduction markers Cx40 and Nav1.5, similar to the precursors of the fast conduction Purkinje fiber network located within the trabeculae of the ventricles. In contrast to the ventricular trabeculae, however, atrial muscle bundles display an increased proliferation rate when compared to the surrounding myocardium. Interestingly, mechanical loading of the embryonic atrial muscle resulted in an induction of Cx40, Nav1.5 and the cell cycle marker Cyclin D1, while decreasing atrial pressure via in vivo ligation of the vitelline blood vessels results in decreased atrial conduction velocity. Taken together, these data establish a novel model for atrial conduction patterning, whereby hemodynamic stretch coordinately induces proliferation and fast conduction marker expression, which in turn promotes the formation of large diameter muscle bundles to serve as preferential routes of conduction.
Conflict of interest statement
Figures
Similar articles
-
Hemodynamic-dependent patterning of endothelin converting enzyme 1 expression and differentiation of impulse-conducting Purkinje fibers in the embryonic heart.Development. 2004 Feb;131(3):581-92. doi: 10.1242/dev.00947. Epub 2004 Jan 7. Development. 2004. PMID: 14711873
-
Developmental regulation of connexin 40 gene expression in mouse heart correlates with the differentiation of the conduction system.Dev Dyn. 1995 Dec;204(4):358-71. doi: 10.1002/aja.1002040403. Dev Dyn. 1995. PMID: 8601030
-
Hemodynamics is a key epigenetic factor in development of the cardiac conduction system.Circ Res. 2003 Jul 11;93(1):77-85. doi: 10.1161/01.RES.0000079488.91342.B7. Epub 2003 May 29. Circ Res. 2003. PMID: 12775585
-
Induction and patterning of the cardiac conduction system.Int J Dev Biol. 2002 Sep;46(6):765-75. Int J Dev Biol. 2002. PMID: 12382942 Review.
-
Developmental transitions in cardiac conduction.Novartis Found Symp. 2003;250:68-75; discussion 76-9, 276-9. Novartis Found Symp. 2003. PMID: 12956324 Review.
Cited by
-
Optical Electrophysiology in the Developing Heart.J Cardiovasc Dev Dis. 2018 May 11;5(2):28. doi: 10.3390/jcdd5020028. J Cardiovasc Dev Dis. 2018. PMID: 29751595 Free PMC article. Review.
-
Distinct mechanisms regulate ventricular and atrial chamber wall formation.Nat Commun. 2024 Sep 17;15(1):8159. doi: 10.1038/s41467-024-52340-3. Nat Commun. 2024. PMID: 39289341 Free PMC article.
-
The in vivo study of cardiac mechano-electric and mechano-mechanical coupling during heart development in zebrafish.Front Physiol. 2023 Mar 16;14:1086050. doi: 10.3389/fphys.2023.1086050. eCollection 2023. Front Physiol. 2023. PMID: 37007999 Free PMC article.
-
Uncovering the Genetic Basis of Congenital Heart Disease: Recent Advancements and Implications for Clinical Management.CJC Pediatr Congenit Heart Dis. 2023 Oct 19;2(6Part B):464-480. doi: 10.1016/j.cjcpc.2023.10.008. eCollection 2023 Dec. CJC Pediatr Congenit Heart Dis. 2023. PMID: 38205435 Free PMC article. Review.
-
Adherens junction engagement regulates functional patterning of the cardiac pacemaker cell lineage.Dev Cell. 2021 May 17;56(10):1498-1511.e7. doi: 10.1016/j.devcel.2021.04.004. Epub 2021 Apr 22. Dev Cell. 2021. PMID: 33891897 Free PMC article.
References
-
- Wenckebach KF (1907) Beiträge zur Kenntnis der menschlichen Herztätigkeit. Virchows Archiv für Pathologische Anatomie und Physiologie und für Klinische Medizin 1–2:1–24.
-
- Thorel C (1910) Uber den Aufbau des Sinusknotens und seine Verbindung mit der Cava superior und den Wenckebachschen Bundeln. Munch Med Wochenschr 57:183–186.
-
- Lewis T, Oppenheimer A, Oppenheimer BS (1910) The site of origin of the mammalian heart beat; the pacemaker in the dog. Heart 2:147–169.
-
- Lewis T, Meakins J, White PD (1914) The excitatory process in the dog's heart. Part I. The auricles. Philosophical Transactions of the Royal Society of London. Series B, Containing Papers of a Biological Character 205:375–420 10.2307/92042 - DOI
-
- Bachmann G (1916) The inter-auricular time interval. Am J Physiol 41:309–320.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

