Protective phenotypes of club cells and alveolar macrophages are favored as part of endotoxin-mediated prevention of asthma
- PMID: 25504013
- PMCID: PMC4935407
- DOI: 10.1177/1535370214562338
Protective phenotypes of club cells and alveolar macrophages are favored as part of endotoxin-mediated prevention of asthma
Abstract
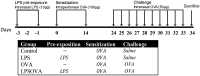
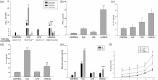
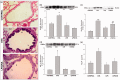
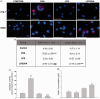
Atopic asthma is a chronic allergic disease that involves T-helper type 2 (Th2)-inflammation and airway remodeling. Bronchiolar club cells (CC) and alveolar macrophages (AM) are sentinel cells of airway barrier against inhaled injuries, where allergy induces mucous metaplasia of CC and the alternative activation of AM, which compromise host defense mechanisms and amplify Th2-inflammation. As there is evidence that high levels of environmental endotoxin modulates asthma, the goal of this study was to evaluate if the activation of local host defenses by Lipopolysaccharide (LPS) previous to allergy development can contribute to preserving CC and AM protective phenotypes. Endotoxin stimulus before allergen exposition reduced hallmarks of allergic inflammation including eosinophil influx, Interleukin-4 and airway hyperreactivity, while the T-helper type 1 related cytokines IL-12 and Interferon-γ were enhanced. This response was accompanied by the preservation of the normal CC phenotype and the anti-allergic proteins Club Cell Secretory Protein (CCSP) and Surfactant-D, thereby leading to lower levels of CC metaplasia and preventing the increase of the pro-Th2 cytokine Thymic stromal lymphopoietin. In addition, classically activated alveolar macrophages expressing nitric oxide were promoted over the alternatively activated ones that expressed arginase-1. We verified that LPS induced a long-term overexpression of CCSP and the innate immune markers Toll-like receptor 4, and Tumor Necrosis Factor-α, changes that were preserved in spite of the allergen challenge. These results demonstrate that LPS pre-exposition modifies the local bronchioalveolar microenvironment by inducing natural anti-allergic mechanisms while reducing local factors that drive Th2 type responses, thus modulating allergic inflammation.
Keywords: Club cell; alveolar macrophage; asthma; pulmonary host defense.
© 2014 by the Society for Experimental Biology and Medicine.
Figures



References
-
- Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald M, Gibson P, Ohta K, O'Byrne P, Pedersen SE, Pizzichini E, Sullivan SD, Wenzel SE, Zar HJ. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J 2008; 31: 143–78. - PubMed
-
- Barnes PJ. Immunology of asthma and chronic obstructive pulmonary disease. Nat Rev Immunol 2008; 8: 183–92. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

