Emerging evidence for specific neuronal functions of auxiliary calcium channel α₂δ subunits
- PMID: 25504062
- PMCID: PMC4487825
- DOI: 10.4149/gpb_2014037
Emerging evidence for specific neuronal functions of auxiliary calcium channel α₂δ subunits
Abstract
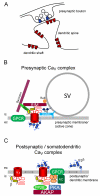
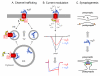
In nerve cells the ubiquitous second messenger calcium regulates a variety of vitally important functions including neurotransmitter release, gene regulation, and neuronal plasticity. The entry of calcium into cells is tightly regulated by voltage-gated calcium channels, which consist of a heteromultimeric complex of a pore forming α₁, and the auxiliary β and α₂δ subunits. Four genes (Cacna2d1-4) encode for the extracellular membrane-attached α₂δ subunits (α₂δ-1 to α₂δ-4), out of which three isoforms (α₂δ-1 to -3) are strongly expressed in the central nervous system. Over the years a wealth of studies has demonstrated the classical role of α₂δ subunits in channel trafficking and calcium current modulation. Recent studies in specialized neuronal cell systems propose roles of α₂δ subunits beyond the classical view and implicate α₂δ subunits as important regulators of synapse formation. These findings are supported by the identification of novel human disease mutations associated with α₂δ subunits and by the fact that α₂δ subunits are the target of the anti-epileptic and anti-allodynic drugs gabapentin and pregabalin. Here we review the recently emerging evidence for specific as well as redundant neuronal roles of α₂δ subunits and discuss the mechanisms for establishing and maintaining specificity.
Figures

Similar articles
-
Pathophysiological Roles of Auxiliary Calcium Channel α2δ Subunits.Handb Exp Pharmacol. 2023;279:289-316. doi: 10.1007/164_2022_630. Handb Exp Pharmacol. 2023. PMID: 36598609
-
Auxiliary subunit regulation of high-voltage activated calcium channels expressed in mammalian cells.Eur J Neurosci. 2004 Jul;20(1):1-13. doi: 10.1111/j.1460-9568.2004.03434.x. Eur J Neurosci. 2004. PMID: 15245474
-
Neurotransmitter modulation of neuronal calcium channels.J Bioenerg Biomembr. 2003 Dec;35(6):477-89. doi: 10.1023/b:jobb.0000008021.55853.18. J Bioenerg Biomembr. 2003. PMID: 15000517 Review.
-
Mechanism of auxiliary subunit modulation of neuronal alpha1E calcium channels.J Gen Physiol. 1998 Aug;112(2):125-43. doi: 10.1085/jgp.112.2.125. J Gen Physiol. 1998. PMID: 9689023 Free PMC article.
-
Emerging Alternative Functions for the Auxiliary Subunits of the Voltage-Gated Calcium Channels.Curr Mol Pharmacol. 2015;8(2):162-8. doi: 10.2174/1874467208666150507110202. Curr Mol Pharmacol. 2015. PMID: 25966689 Review.
Cited by
-
NRL and CRX Define Photoreceptor Identity and Reveal Subgroup-Specific Dependencies in Medulloblastoma.Cancer Cell. 2018 Mar 12;33(3):435-449.e6. doi: 10.1016/j.ccell.2018.02.006. Cancer Cell. 2018. PMID: 29533784 Free PMC article.
-
Presynaptic α2δ-2 Calcium Channel Subunits Regulate Postsynaptic GABAA Receptor Abundance and Axonal Wiring.J Neurosci. 2019 Apr 3;39(14):2581-2605. doi: 10.1523/JNEUROSCI.2234-18.2019. Epub 2019 Jan 25. J Neurosci. 2019. PMID: 30683685 Free PMC article.
-
Genetic Associations between Voltage-Gated Calcium Channels and Psychiatric Disorders.Int J Mol Sci. 2019 Jul 19;20(14):3537. doi: 10.3390/ijms20143537. Int J Mol Sci. 2019. PMID: 31331039 Free PMC article. Review.
-
Voltage-gated calcium channel α 2δ subunits: an assessment of proposed novel roles.F1000Res. 2018 Nov 21;7:F1000 Faculty Rev-1830. doi: 10.12688/f1000research.16104.1. eCollection 2018. F1000Res. 2018. PMID: 30519455 Free PMC article. Review.
-
α2δ2 Controls the Function and Trans-Synaptic Coupling of Cav1.3 Channels in Mouse Inner Hair Cells and Is Essential for Normal Hearing.J Neurosci. 2016 Oct 26;36(43):11024-11036. doi: 10.1523/JNEUROSCI.3468-14.2016. J Neurosci. 2016. PMID: 27798183 Free PMC article.
References
-
- Anantharaman V, Aravind L. Cache - a signaling domain common to animal Ca(2+)-channel subunits and a class of prokaryotic chemotaxis receptors. Trends Biochem. Sci. 2000;25:535–537. - PubMed
-
- Arikkath J, Campbell KP. Auxiliary subunits: essential components of the voltage-gated calcium channel complex. Curr. Opin. Neurobiol. 2003;13:298–307. - PubMed
-
- Barclay J, Balaguero N, Mione M, Ackerman SL, Letts VA, Brodbeck J, Canti C, Meir A, Page KM, Kusumi K, et al. Ducky mouse phenotype of epilepsy and ataxia is associated with mutations in the Cacna2d2 gene and decreased calcium channel current in cerebellar Purkinje cells. J. Neurosci. 2001;21:6095–6104. - PMC - PubMed
-
- Bauer CS, Nieto-Rostro M, Rahman W, Tran-Van-Minh A, Ferron L, Douglas L, Kadurin I, Sri Ranjan Y, Fernandez-Alacid L, Millar NS, et al. The increased trafficking of the calcium channel subunit alpha2delta-1 to presynaptic terminals in neuropathic pain is inhibited by the alpha2delta ligand pregabalin. J. Neurosci. 2009;29:4076–4088. - PMC - PubMed
-
- Bauer CS, Tran-Van-Minh A, Kadurin I, Dolphin AC. A new look at calcium channel alpha2delta subunits. Curr. Opin. Neurobiol. 2010;20:563–571. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
