Long-Term Gestational Hypoxia Modulates Expression of Key Genes Governing Mitochondrial Function in the Perirenal Adipose of the Late Gestation Sheep Fetus
- PMID: 25504105
- PMCID: PMC4502806
- DOI: 10.1177/1933719114561554
Long-Term Gestational Hypoxia Modulates Expression of Key Genes Governing Mitochondrial Function in the Perirenal Adipose of the Late Gestation Sheep Fetus
Abstract
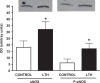
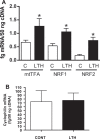
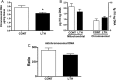
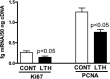
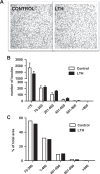
We previously reported that long-term hypoxia (LTH) increases expression of brown adipose tissue (BAT) genes in the perirenal adipose in the ovine fetus. The mechanisms with which hypoxia mediates the enhanced BAT phenotype are unresolved. This study was designed to examine the effects of LTH on (1) the expression of endothelial cell nitric oxide synthase (eNOS) and (2) indicators of mitochondrial biogenesis (transcription factors mitochondrial transcription factor A (mtTFA), nuclear respiratory factor (NRF) 1, and NRF-2; cytochrome c oxidase (COX) I, II, and IV and mitochondrial DNA content). Pregnant ewes were maintained at high altitude (3820 m) from ∼40 to 137 to 140 days of gestation and perirenal adipose was collected from normoxic control and LTH fetuses. There was no effect of LTH on fetal body weight or perirenal adipose mass. Long-term hypoxia increased (P < .05) perirenal eNOS and phospho-eNOS, messenger RNA (mRNA) for NRF1, NRF-2, mtTFA as well as COX-I, COX-II, and COX-IV mRNA. In contrast, mRNA for 2 markers for cellular proliferation (Ki67 and proliferating cell nuclear antigen [PCNA]) was lower in perirenal adipose from LTH fetuses compared to controls (P < .05), while mitochondrial to nuclear DNA ratio did not differ between groups. In conclusion, nitric oxide may function as a mechanism via which LTH enhances the BAT phenotype in fetal sheep prior to birth. Although there is an apparent increase in genes supporting mitochondrial function and adaptive thermogenesis in response to LTH, there does not appear to be an increased mitochondrial biogenesis per se. Such adaptive changes may provide a mechanism for the prominence of the BAT phenotype observed in the late gestation LTH fetus.
Keywords: adipose; fetus; hypoxia; mitochondria; ovine; sheep.
© The Author(s) 2014.
Conflict of interest statement
Figures


Similar articles
-
Long-term hypoxia modulates expression of key genes regulating adipose function in the late-gestation ovine fetus.Am J Physiol Regul Integr Comp Physiol. 2008 Apr;294(4):R1312-8. doi: 10.1152/ajpregu.00004.2008. Epub 2008 Feb 20. Am J Physiol Regul Integr Comp Physiol. 2008. PMID: 18287225
-
Adrenocorticotropic Hormone and PI3K/Akt Inhibition Reduce eNOS Phosphorylation and Increase Cortisol Biosynthesis in Long-Term Hypoxic Ovine Fetal Adrenal Cortical Cells.Reprod Sci. 2015 Aug;22(8):932-41. doi: 10.1177/1933719115570899. Epub 2015 Feb 5. Reprod Sci. 2015. PMID: 25656500 Free PMC article.
-
Recombinant human ciliary neurotrophic factor reduces weight partly by regulating nuclear respiratory factor 1 and mitochondrial transcription factor A.Eur J Pharmacol. 2007 Jun 1;563(1-3):77-82. doi: 10.1016/j.ejphar.2007.02.034. Epub 2007 Feb 27. Eur J Pharmacol. 2007. PMID: 17397829
-
Adrenocortical and adipose responses to high-altitude-induced, long-term hypoxia in the ovine fetus.J Pregnancy. 2012;2012:681306. doi: 10.1155/2012/681306. Epub 2012 May 14. J Pregnancy. 2012. PMID: 22666594 Free PMC article. Review.
-
Fetal endocrine and metabolic adaptations to hypoxia: the role of the hypothalamic-pituitary-adrenal axis.Am J Physiol Endocrinol Metab. 2015 Sep 1;309(5):E429-39. doi: 10.1152/ajpendo.00126.2015. Epub 2015 Jul 14. Am J Physiol Endocrinol Metab. 2015. PMID: 26173460 Free PMC article. Review.
Cited by
-
Gestational Hypoxia and Programing of Lung Metabolism.Front Physiol. 2019 Nov 29;10:1453. doi: 10.3389/fphys.2019.01453. eCollection 2019. Front Physiol. 2019. PMID: 31849704 Free PMC article. Review.
-
Role of placental insufficiency and intrauterine growth restriction on the activation of fetal hepatic glucose production.Mol Cell Endocrinol. 2016 Nov 5;435:61-68. doi: 10.1016/j.mce.2015.12.016. Epub 2015 Dec 23. Mol Cell Endocrinol. 2016. PMID: 26723529 Free PMC article.
-
Preeclampsia link to gestational hypoxia.J Dev Orig Health Dis. 2019 Jun;10(3):322-333. doi: 10.1017/S204017441900014X. Epub 2019 Apr 10. J Dev Orig Health Dis. 2019. PMID: 30968806 Free PMC article. Review.
References
-
- Pope M, Budge H, Symonds ME. The developmental transition of ovine adipose tissue through early life. Acta Physiol (Oxf). 2014;210 (1):20–30. - PubMed
-
- Poissonnet CM, Burdi AR, Garn SM. The chronology of adipose tissue appearance and distribution in the human fetus. Early Hum Dev. 1984;10 (1-2):1–11. - PubMed
-
- Clarke L, Buss DS, Juniper DT, Lomax MA, Symonds ME. Adipose tissue development during early postnatal life in ewe-reared lambs. Exp Physiol. 1997;82 (6):1015–1027. - PubMed
-
- Cannon B, Nedergaard JAN. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84 (1):277–359. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

