Covalent modification of a cysteine residue in the XPB subunit of the general transcription factor TFIIH through single epoxide cleavage of the transcription inhibitor triptolide
- PMID: 25504624
- PMCID: PMC4314353
- DOI: 10.1002/anie.201408817
Covalent modification of a cysteine residue in the XPB subunit of the general transcription factor TFIIH through single epoxide cleavage of the transcription inhibitor triptolide
Abstract
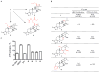
Triptolide is a key component of the traditional Chinese medicinal plant Thunder God Vine and has potent anticancer and immunosuppressive activities. It is an irreversible inhibitor of eukaryotic transcription through covalent modification of XPB, a subunit of the general transcription factor TFIIH. Cys342 of XPB was identified as the residue that undergoes covalent modification by the 12,13-epoxide group of triptolide. Mutation of Cys342 of XPB to threonine conferred resistance to triptolide on the mutant protein. Replacement of the endogenous wild-type XPB with the Cys342Thr mutant in a HEK293T cell line rendered it completely resistant to triptolide, thus validating XPB as the physiologically relevant target of triptolide. Together, these results deepen our understanding of the interaction between triptolide and XPB and have implications for the future development of new analogues of triptolide as leads for anticancer and immunosuppressive drugs.
Keywords: inhibitors; medicinal chemistry; natural products; target validation; transcription factors.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures
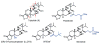


Similar articles
-
XPB, a subunit of TFIIH, is a target of the natural product triptolide.Nat Chem Biol. 2011 Mar;7(3):182-8. doi: 10.1038/nchembio.522. Epub 2011 Jan 30. Nat Chem Biol. 2011. PMID: 21278739 Free PMC article.
-
The essential and multifunctional TFIIH complex.Protein Sci. 2018 Jun;27(6):1018-1037. doi: 10.1002/pro.3424. Epub 2018 Apr 27. Protein Sci. 2018. PMID: 29664212 Free PMC article. Review.
-
Targeted Delivery and Sustained Antitumor Activity of Triptolide through Glucose Conjugation.Angew Chem Int Ed Engl. 2016 Sep 19;55(39):12035-9. doi: 10.1002/anie.201606121. Epub 2016 Aug 30. Angew Chem Int Ed Engl. 2016. PMID: 27574181
-
Transcription without XPB Establishes a Unified Helicase-Independent Mechanism of Promoter Opening in Eukaryotic Gene Expression.Mol Cell. 2017 Feb 2;65(3):504-514.e4. doi: 10.1016/j.molcel.2017.01.012. Mol Cell. 2017. PMID: 28157507
-
A history of TFIIH: two decades of molecular biology on a pivotal transcription/repair factor.DNA Repair (Amst). 2011 Jul 15;10(7):714-21. doi: 10.1016/j.dnarep.2011.04.021. Epub 2011 May 17. DNA Repair (Amst). 2011. PMID: 21592869 Review.
Cited by
-
Probing Dual Covalent Irreversible Inhibition of EGFR/FGFR4 by Electrophilic-Based Natural Compounds to Overcome Resistance and Enhance Combination Therapeutic Potentials and Management of Hepatocellular Carcinoma (HCC).Protein J. 2024 Aug;43(4):793-804. doi: 10.1007/s10930-024-10211-2. Epub 2024 Jul 9. Protein J. 2024. PMID: 38981944
-
ATR Kinase Activity Limits Mutagenesis and Promotes the Clonogenic Survival of Quiescent Human Keratinocytes Exposed to UVB Radiation.Photochem Photobiol. 2020 Jan;96(1):105-112. doi: 10.1111/php.13164. Epub 2019 Oct 17. Photochem Photobiol. 2020. PMID: 31554014 Free PMC article.
-
NAD (P)H Quinone Dehydrogenase 1-Targeting Triptolide Analogue Causes Tumor Regression and Sensitizes Cisplatin-Resistant Lung Cancer to Chemotherapy.ACS Pharmacol Transl Sci. 2023 Sep 6;6(10):1508-1517. doi: 10.1021/acsptsci.3c00144. eCollection 2023 Oct 13. ACS Pharmacol Transl Sci. 2023. PMID: 37854615 Free PMC article.
-
Antitumor mechanisms and future clinical applications of the natural product triptolide.Cancer Cell Int. 2024 Apr 27;24(1):150. doi: 10.1186/s12935-024-03336-y. Cancer Cell Int. 2024. PMID: 38678240 Free PMC article. Review.
-
RACK7 senses and fine-tunes enhancer activity.iScience. 2025 Jul 9;28(8):113083. doi: 10.1016/j.isci.2025.113083. eCollection 2025 Aug 15. iScience. 2025. PMID: 40734674 Free PMC article.
References
-
- Kupchan SM, Court WA, Dailey RG, Jr, Gilmore CJ, Bryan RF. J Am Chem Soc. 1972;94:7194–7195. - PubMed
- Zhao X-M. In: Supplement to Materia Medica. Xing Zhang’s Jie., editor. Tang Pulishing House; Qian Tang: 1765.
-
- Qiu D, Zhao G, Aoki Y, Shi L, Uyei A, Nazarian S, Ng JC, Kao PN. J Biol Chem. 1999;274:13443–13450. - PubMed
- Westerheide SD, Kawahara TL, Orton K, Morimoto RI. J Biol Chem. 2006;281:9616–9622. - PubMed
- Lee KY, Chang W, Qiu D, Kao PN, Rosen GD. J Biol Chem. 1999;274:13451–13455. - PubMed
- Leuenroth SJ, Crews CM. Cancer Res. 2008;68:5257–5266. - PMC - PubMed
- McCallum C, Kwon S, Leavitt P, Shoop W, Michael B, Felcetto T, Zaller D, O’Neill E, Frantz-Wattley B, Thompson C, Forrest G, Carballo-Jane E, Gurnett A. Therapy. 2005;2:261–273.
- Vispe S, DeVries L, Creancier L, Besse J, Breand S, Hobson DJ, Svejstrup JQ, Annereau JP, Cussac D, Dumontet C, Guilbaud N, Barret JM, Bailly C. Mol Cancer Ther. 2009;8:2780–2790. - PubMed
-
- Leuenroth SJ, Okuhara D, Shotwell JD, Markowitz GS, Yu Z, Somlo S, Crews CM. Proc Natl Acad Sci USA. 2007;104:4389–4394. - PMC - PubMed
- Soundararajan R, Sayat R, Robertson GS, Marignani PA. Cancer Biol Ther. 2009;8:2054–2062. - PubMed
- Corson TW, Cavga H, Aberle N, Crews CM. Chem Bio Chem. 2011;12:1767–1773. - PMC - PubMed
- Lu Y, Zhang Y, Li L, Feng X, Ding S, Zheng W, Li J, Shen P. Chem Biol. 2014;21:246–256. - PubMed
-
- Zhou ZL, Yang YX, Ding J, Li YC, Miao ZH. Nat Prod Rep. 2012;29:457–475. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

