Convergent transcriptional specializations in the brains of humans and song-learning birds
- PMID: 25504733
- PMCID: PMC4385736
- DOI: 10.1126/science.1256846
Convergent transcriptional specializations in the brains of humans and song-learning birds
Abstract
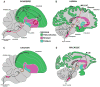
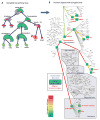
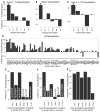
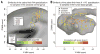
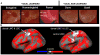
Song-learning birds and humans share independently evolved similarities in brain pathways for vocal learning that are essential for song and speech and are not found in most other species. Comparisons of brain transcriptomes of song-learning birds and humans relative to vocal nonlearners identified convergent gene expression specializations in specific song and speech brain regions of avian vocal learners and humans. The strongest shared profiles relate bird motor and striatal song-learning nuclei, respectively, with human laryngeal motor cortex and parts of the striatum that control speech production and learning. Most of the associated genes function in motor control and brain connectivity. Thus, convergent behavior and neural connectivity for a complex trait are associated with convergent specialized expression of multiple genes.
Copyright © 2014, American Association for the Advancement of Science.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

