Ectokinases as novel cancer markers and drug targets in cancer therapy
- PMID: 25504773
- PMCID: PMC4380966
- DOI: 10.1002/cam4.368
Ectokinases as novel cancer markers and drug targets in cancer therapy
Abstract
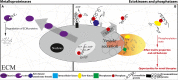
While small-molecule kinase inhibitors became the most prominent anticancer drugs, novel combinatorial strategies need to be developed as the fight against cancer is not yet won. We review emerging literature showing that the release of several ectokinases is significantly upregulated in body fluids from cancer patients and that they leave behind their unique signatures on extracellular matrix (ECM) proteins. Our analysis of proteomic data reveals that fibronectin is heavily phosphorylated in cancer tissues particularly within its growth factor binding sites and on domains that regulate fibrillogenesis. We are thus making the case that cancer is not only a disease of cells but also of the ECM. Targeting extracellular kinases or the extracellular signatures they leave behind might thus create novel opportunities in cancer diagnosis as well as new avenues to interfere with cancer progression and malignancy.
Keywords: Cancer marker; drug design; ectokinases; exokinases; extracellular matrix; extracellular phosphorylation; extracellular protein kinase; personalized medicine.
© 2014 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Protein Kinases as Tumor Biomarkers and Therapeutic Targets.Curr Pharm Des. 2017 Nov 16;23(29):4209-4225. doi: 10.2174/1381612823666170720113216. Curr Pharm Des. 2017. PMID: 28730960 Review.
-
Promises and drawbacks of targeting cell cycle kinases in cancer.Discov Med. 2009 Dec;8(43):177-80. Discov Med. 2009. PMID: 20040266 Review.
-
New paradigms in anticancer therapy: targeting multiple signaling pathways with kinase inhibitors.Semin Oncol. 2006 Aug;33(4):407-20. doi: 10.1053/j.seminoncol.2006.04.005. Semin Oncol. 2006. PMID: 16890796 Review.
-
Novel approaches to the development of tyrosine kinase inhibitors and their role in the fight against cancer.Expert Opin Drug Discov. 2014 Jan;9(1):77-92. doi: 10.1517/17460441.2014.865012. Epub 2013 Dec 3. Expert Opin Drug Discov. 2014. PMID: 24294890 Review.
-
MKK3 as oncotarget.Aging (Albany NY). 2016 Jan;8(1):1-2. doi: 10.18632/aging.100878. Aging (Albany NY). 2016. PMID: 26805700 Free PMC article. No abstract available.
Cited by
-
Proteomic database mining opens up avenues utilizing extracellular protein phosphorylation for novel therapeutic applications.J Transl Med. 2015 Apr 19;13:125. doi: 10.1186/s12967-015-0482-4. J Transl Med. 2015. PMID: 25927841 Free PMC article. Review.
-
CK2 and the Hallmarks of Cancer.Biomedicines. 2022 Aug 16;10(8):1987. doi: 10.3390/biomedicines10081987. Biomedicines. 2022. PMID: 36009534 Free PMC article. Review.
-
Concepts of extracellular matrix remodelling in tumour progression and metastasis.Nat Commun. 2020 Oct 9;11(1):5120. doi: 10.1038/s41467-020-18794-x. Nat Commun. 2020. PMID: 33037194 Free PMC article. Review.
-
Vertebrate Lonesome Kinase Regulated Extracellular Matrix Protein Phosphorylation, Cell Shape, and Adhesion in Trabecular Meshwork Cells.J Cell Physiol. 2017 Sep;232(9):2447-2460. doi: 10.1002/jcp.25582. Epub 2017 Mar 27. J Cell Physiol. 2017. PMID: 27591737 Free PMC article.
-
The Extracellular Matrix: Its Composition, Function, Remodeling, and Role in Tumorigenesis.Biomimetics (Basel). 2023 Apr 5;8(2):146. doi: 10.3390/biomimetics8020146. Biomimetics (Basel). 2023. PMID: 37092398 Free PMC article. Review.
References
-
- Lippert TH, Ruoff HJ. Volm M. Intrinsic and acquired drug resistance in malignant tumors. The main reason for therapeutic failure. Arzneimittelforschung. 2008;58:261–264. - PubMed
-
- Gottesman MM. Mechanisms of cancer drug resistance. Annu. Rev. Med. 2002;53:615–627. - PubMed
-
- Santarpia M, De Pas TM, Altavilla G, Spaggiari L. Rosell R. Moving towards molecular-guided treatments: erlotinib and clinical outcomes in non-small-cell lung cancer patients. Future Oncol. 2013;9:327–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

