Chronic fetal hypoxia affects axonal maturation in guinea pigs during development: A longitudinal diffusion tensor imaging and T2 mapping study
- PMID: 25504885
- PMCID: PMC4468050
- DOI: 10.1002/jmri.24825
Chronic fetal hypoxia affects axonal maturation in guinea pigs during development: A longitudinal diffusion tensor imaging and T2 mapping study
Abstract
Purpose: To investigate the impact of chronic hypoxia on neonatal brains, and follow developmental alterations and adaptations noninvasively in a guinea pig model. Chronic hypoxemia is the prime cause of fetal brain injury and long-term sequelae such as neurodevelopmental compromise, seizures, and cerebral palsy.
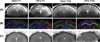
Materials and methods: Thirty guinea pigs underwent either normoxic and hypoxemic conditions during the critical stage of brain development (0.7 gestation) and studied prenatally (n = 16) or perinatally (n = 14). Fourteen newborns (7 hypoxia and 7 normoxia group) were scanned longitudinally to characterize physiological and morphological alterations, and axonal myelination and injury using in vivo diffusion tensor imaging (DTI), T2 mapping, and T2 -weighted magnetic resonance imaging (MRI). Sixteen fetuses (8 hypoxia and 8 normoxia) were studied ex vivo to assess hypoxia-induced neuronal injury/loss using Nissl staining and quantitative reverse transcriptase polymerase chain reaction methods.
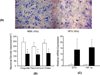
Results: Developmental brains in the hypoxia group showed lower fractional anisotropy in the corpus callosum (-12%, P = 0.02) and lower T2 values in the hippocampus (-16%, P = 0.003) compared with the normoxia group with no differences in the cortex (P > 0.07), indicating vulnerability of the hippocampus and cerebral white matter during early development. Fetal guinea pig brains with chronic hypoxia demonstrated an over 10-fold increase in expression levels of hypoxia index genes such as erythropoietin and HIF-1α, and an over 40% reduction in neuronal density, confirming prenatal brain damage.
Conclusion: In vivo MRI measurement, such as DTI and T2 mapping, provides quantitative parameters to characterize neurodevelopmental abnormalities and to monitor the impact of prenatal insult on the postnatal brain maturation of guinea pigs.
Keywords: DTI; T2; brain development; fetal hypoxia; guinea pig; prenatal brain injury.
© 2014 Wiley Periodicals, Inc.
Figures



References
-
- Holling EE, Leviton A. Characteristics of cranial ultrasound white-matter echolucencies that predict disability: a review. Dev Med Child Neurol. 1999;41:136–139. - PubMed
-
- Platt MJ, Cans C, Johnson A, et al. Trends in cerebral palsy among infants of very low birthweight (<1500g) or born prematurely (<32 weeks) in 16 European centres: a database study. Lancet. 2007;369:43–50. - PubMed
-
- Allin M, Walshe M, Fern A, et al. Cognitive maturation in preterm and term born adolescents. J Neurol Neurosurg Psychiatr. 2008;79:381–386. - PubMed
-
- Larroque B, Ancel P-Y, Marret S, et al. Neurodevelopmental disabilities and special care of 5-year-old children born before 33 weeks of gestation (the EPIPAGE study): a longitudinal cohort study. Lancet. 2008;371:813–820. - PubMed
-
- Woodward LJ, Edgin JO, Thompson D, Inder TE. Object working memory deficits predicted by early brain injury and development in the preterm infant. Brain. 2005;128:2578–2587. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

