Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors
- PMID: 25504959
- PMCID: PMC4472310
- DOI: 10.1002/art.38990
Myofibroblasts in murine cutaneous fibrosis originate from adiponectin-positive intradermal progenitors
Abstract
Objective: Accumulation of myofibroblasts in fibrotic skin is a hallmark of systemic sclerosis (SSc; scleroderma), but the origins of these cells remain unknown. Because loss of intradermal adipose tissue is a consistent feature of cutaneous fibrosis, we sought to examine the hypothesis that myofibroblasts populating fibrotic dermis derive from adipocytic progenitors.
Methods: We performed genetic fate mapping studies to investigate the loss of intradermal adipose tissue and its potential role in fibrosis in mice with bleomycin-induced scleroderma. Modulation of adipocytic phenotypes ex vivo was investigated in adipose tissue-derived cells in culture.
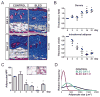
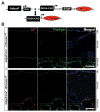
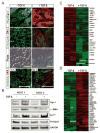
Results: A striking loss of intradermal adipose tissue and its replacement with fibrous tissue were consistently observed in mice with bleomycin-induced fibrosis. Loss of adipose tissue and a decline in the expression of canonical adipogenic markers in lesional skin preceded the onset of dermal fibrosis and expression of fibrogenic markers. Ex vivo, subcutaneous adipocytes were driven by transforming growth factor β to preferentially undergo fibrogenic differentiation. Cell fate mapping studies in mice with the adiponectin promoter-driven Cre recombinase transgenic construct indicated that adiponectin-positive progenitors that are normally confined to the intradermal adipose tissue compartment were distributed throughout the lesional dermis over time, lost their adipocytic markers, and expressed myofibroblast markers in bleomycin-treated mice.
Conclusion: These observations establish a novel link between intradermal adipose tissue loss and dermal fibrosis and demonstrate that adiponectin-positive intradermal progenitors give rise to dermal myofibroblasts. Adipose tissue loss and adipocyte-myofibroblast transition might be primary events in the pathogenesis of cutaneous fibrosis that represent novel potential targets for therapeutic intervention.
Copyright © 2015 by the American College of Rheumatology.
Figures



Comment in
-
Editorial: Fate of fat tissue adipocytes: do they transform into myofibroblasts in scleroderma?Arthritis Rheumatol. 2015 Apr;67(4):860-1. doi: 10.1002/art.38988. Arthritis Rheumatol. 2015. PMID: 25504912 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
- CA-060553/CA/NCI NIH HHS/United States
- R01 AR042309/AR/NIAMS NIH HHS/United States
- P30-CA-060553/CA/NCI NIH HHS/United States
- R56 AR042309/AR/NIAMS NIH HHS/United States
- K26 OD010945/OD/NIH HHS/United States
- P30 CA060553/CA/NCI NIH HHS/United States
- AR-42309/AR/NIAMS NIH HHS/United States
- K12 HD055884/HD/NICHD NIH HHS/United States
- P30-AR-061271/AR/NIAMS NIH HHS/United States
- T32-AR-060710/AR/NIAMS NIH HHS/United States
- OD-010945/OD/NIH HHS/United States
- T32 AR060710/AR/NIAMS NIH HHS/United States
- P30 AR061271/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

