Dengue virus induces and requires glycolysis for optimal replication
- PMID: 25505078
- PMCID: PMC4338897
- DOI: 10.1128/JVI.02309-14
Dengue virus induces and requires glycolysis for optimal replication
Abstract
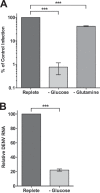
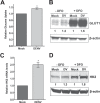
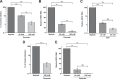
Viruses rely on host cellular metabolism to provide the energy and biosynthetic building blocks required for their replication. Dengue virus (DENV), a member of the Flaviviridae family, is one of the most important arthropod-borne human pathogens worldwide. We analyzed global intracellular metabolic changes associated with DENV infection of primary human cells. Our metabolic profiling data suggested that central carbon metabolism, particularly glycolysis, is strikingly altered during a time course of DENV infection. Glucose consumption is increased during DENV infection and depriving DENV-infected cells of exogenous glucose had a pronounced impact on viral replication. Furthermore, the expression of both glucose transporter 1 and hexokinase 2, the first enzyme of glycolysis, is upregulated in DENV-infected cells. Pharmacologically inhibiting the glycolytic pathway dramatically reduced DENV RNA synthesis and infectious virion production, revealing a requirement for glycolysis during DENV infection. Thus, these experiments suggest that DENV induces the glycolytic pathway to support efficient viral replication. This study raises the possibility that metabolic inhibitors, such as those that target glycolysis, could be used to treat DENV infection in the future.
Importance: Approximately 400 million people are infected with dengue virus (DENV) annually, and more than one-third of the global population is at risk of infection. As there are currently no effective vaccines or specific antiviral therapies for DENV, we investigated the impact DENV has on the host cellular metabolome to identify metabolic pathways that are critical for the virus life cycle. We report an essential role for glycolysis during DENV infection. DENV activates the glycolytic pathway, and inhibition of glycolysis significantly blocks infectious DENV production. This study provides further evidence that viral metabolomic analyses can lead to the discovery of novel therapeutic targets to block the replication of medically important human pathogens.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures

References
-
- Diamond DL, Syder AJ, Jacobs JM, Sorensen CM, Walters KA, Proll SC, McDermott JE, Gritsenko MA, Zhang Q, Zhao R, Metz TO, Camp DG II, Waters KM, Smith RD, Rice CM, Katze MG. 2010. Temporal proteome and lipidome profiles reveal hepatitis C virus-associated reprogramming of hepatocellular metabolism and bioenergetics. PLoS Pathog 6:e1000719. doi:10.1371/journal.ppat.1000719. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

