Dendritic spine dysgenesis contributes to hyperreflexia after spinal cord injury
- PMID: 25505110
- PMCID: PMC4346729
- DOI: 10.1152/jn.00566.2014
Dendritic spine dysgenesis contributes to hyperreflexia after spinal cord injury
Abstract
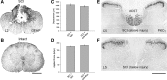
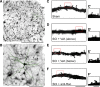
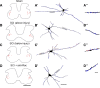
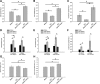
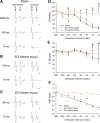
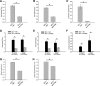
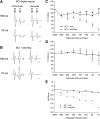
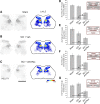
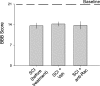
Hyperreflexia and spasticity are chronic complications in spinal cord injury (SCI), with limited options for safe and effective treatment. A central mechanism in spasticity is hyperexcitability of the spinal stretch reflex, which presents symptomatically as a velocity-dependent increase in tonic stretch reflexes and exaggerated tendon jerks. In this study we tested the hypothesis that dendritic spine remodeling within motor reflex pathways in the spinal cord contributes to H-reflex dysfunction indicative of spasticity after contusion SCI. Six weeks after SCI in adult Sprague-Dawley rats, we observed changes in dendritic spine morphology on α-motor neurons below the level of injury, including increased density, altered spine shape, and redistribution along dendritic branches. These abnormal spine morphologies accompanied the loss of H-reflex rate-dependent depression (RDD) and increased ratio of H-reflex to M-wave responses (H/M ratio). Above the level of injury, spine density decreased compared with below-injury spine profiles and spine distributions were similar to those for uninjured controls. As expected, there was no H-reflex hyperexcitability above the level of injury in forelimb H-reflex testing. Treatment with NSC23766, a Rac1-specific inhibitor, decreased the presence of abnormal dendritic spine profiles below the level of injury, restored RDD of the H-reflex, and decreased H/M ratios in SCI animals. These findings provide evidence for a novel mechanistic relationship between abnormal dendritic spine remodeling in the spinal cord motor system and reflex dysfunction in SCI.
Keywords: H-reflex; Rac1; hyperreflexia; spasticity; spinal cord injury.
Figures
References
-
- Alvarez FJ, Villalba RM, Zerda R, Schneider SP. Vesicular glutamate transporters in the spinal cord, with special reference to sensory primary afferent synapses. J Comp Neurol 472: 257–280, 2004. - PubMed
-
- Anderson LC, von Bartheld CS, Byers MR. NGF depletion reduces ipsilateral and contralateral trigeminal satellite cell reactions after inferior alveolar nerve injury in adult rats. Exp Neurol 150: 312–320, 1998. - PubMed
-
- Arendt-Nielsen L, Graven-Nielsen T, Svensson P, Jensen TS. Temporal summation in muscles and referred pain areas: an experimental human study. Muscle Nerve 20: 1311–1313, 1997. - PubMed
-
- Arendt-Nielsen L, Sonnenborg FA, Andersen OK. Facilitation of the withdrawal reflex by repeated transcutaneous electrical stimulation: an experimental study on central integration in humans. Eur J Appl Physiol 81: 165–173, 2000. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

