A proteomic analysis reveals that Snail regulates the expression of the nuclear orphan receptor Nuclear Receptor Subfamily 2 Group F Member 6 (Nr2f6) and interleukin 17 (IL-17) to inhibit adipocyte differentiation
- PMID: 25505127
- PMCID: PMC4350027
- DOI: 10.1074/mcp.M114.045328
A proteomic analysis reveals that Snail regulates the expression of the nuclear orphan receptor Nuclear Receptor Subfamily 2 Group F Member 6 (Nr2f6) and interleukin 17 (IL-17) to inhibit adipocyte differentiation
Abstract
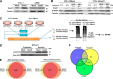
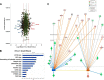
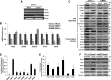
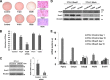
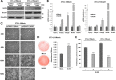
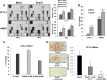
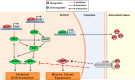
Adipogenesis requires a differentiation program driven by multiple transcription factors, where PPARγ and C/EBPα play a central role. Recent findings indicate that Snail inhibits adipocyte differentiation in 3T3-L1 and murine mesenchymal stem cells (mMSC). An in-depth quantitative SILAC analysis of the nuclear fraction of Snail-induced alterations of 3T3-L1 cells was carried out. In total, 2251 overlapping proteins were simultaneously quantified in forward and reverse experiments. We observed 574 proteins deregulated by Snail1 using a fold-change ≥1.5, with 111 up- and 463 down-regulated proteins, respectively. Among other proteins, multiple transcription factors such as Trip4, OsmR, Nr2f6, Cbx6, and Prrx1 were down-regulated. Results were validated in 3T3-L1 cells and mMSC cells by Western blot and quantitative PCR. Knock-down experiments in 3T3-L1 cells demonstrated that only Nr2f6 (and Trip4 at minor extent) was required for adipocyte differentiation. Ectopic expression of Nr2f6 reversed the effects of Snail1 and promoted adipogenesis. Because Nr2f6 inhibits the expression of IL-17, we tested the effect of Snail on IL-17 expression. IL-17 and TNFα were among the most up-regulated pro-inflammatory cytokines in Snail-transfected 3T3-L1 and mMSC cells. Furthermore, the blocking of IL-17 activity in Snail-transfected cells promoted adipocyte differentiation, reverting Snail inhibition. In summary, Snail inhibits adipogenesis through a down-regulation of Nr2f6, which in turn facilitates the expression of IL-17, an anti-adipogenic cytokine. These results would support a novel and important role for Snail and Nr2f6 in obesity control.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
References
-
- Freytag S. O., Paielli D. L., Gilbert J. D. (1994) Ectopic expression of the CCAAT/enhancer-binding protein alpha promotes the adipogenic program in a variety of mouse fibroblastic cells. Genes Dev. 8, 1654–1663 - PubMed
-
- Tontonoz P., Hu E., Spiegelman B. M. (1994) Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79, 1147–1156 - PubMed
-
- Barrallo-Gimeno A., Nieto M. A. (2005) The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132, 3151–3161 - PubMed
-
- Peinado H., Olmeda D., Cano A. (2007) Snail, Zeb, and bHLH factors in tumor progression: an alliance against the epithelial phenotype? Nat. Rev. Cancer 7, 415–428 - PubMed
-
- Franci C., Takkunen M., Dave N., Alameda F., Gomez S., Rodriguez R., Escriva M., Montserrat-Sentis B., Baro T., Garrido M., Bonilla F., Virtanen I., Garcia de Herreros A. (2006) Expression of Snail protein in tumor-stroma interface. Oncogene 25, 5134–5144 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

