Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells
- PMID: 25505184
- PMCID: PMC4319033
- DOI: 10.1074/jbc.M114.611210
Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells
Abstract
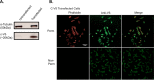
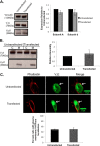
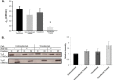
The vacuolar (H(+))-ATPases (V-ATPases) are a family of ATP-driven proton pumps that couple ATP hydrolysis with translocation of protons across membranes. Previous studies have implicated V-ATPases in cancer cell invasion. It has been proposed that V-ATPases participate in invasion by localizing to the plasma membrane and causing acidification of the extracellular space. To test this hypothesis, we utilized two separate approaches to specifically inhibit plasma membrane V-ATPases. First, we stably transfected highly invasive MDA-MB231 cells with a V5-tagged construct of the membrane-embedded c subunit of the V-ATPase, allowing for extracellular expression of the V5 epitope. We evaluated the effect of addition of a monoclonal antibody directed against the V5 epitope on both V-ATPase-mediated proton translocation across the plasma membrane and invasion using an in vitro Matrigel assay. The addition of anti-V5 antibody resulted in acidification of the cytosol and a decrease in V-ATPase-dependent proton flux across the plasma membrane in transfected but not control (untransfected) cells. These results demonstrate that the anti-V5 antibody inhibits activity of plasma membrane V-ATPases in transfected cells. Addition of the anti-V5 antibody also inhibited in vitro invasion of transfected (but not untransfected) cells. Second, we utilized a biotin-conjugated form of the specific V-ATPase inhibitor bafilomycin. When bound to streptavidin, this compound cannot cross the plasma membrane. Addition of this compound to MDA-MB231 cells also inhibited in vitro invasion. These studies suggest that plasma membrane V-ATPases play an important role in invasion of breast cancer cells.
Keywords: Acidification; Breast Cancer; Invasion; Migration; Proton Transport; Vacuolar ATPase.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
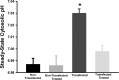

References
-
- Nguyen D. X., Bos P. D., Massagué J. (2009) Metastasis: from dissemination to organ-specific colonization. Nat. Rev. Cancer 9, 274–284 - PubMed
-
- Spano D., Zollo M. (2012) Tumor microenvironment: a main actor in the metastasis process. Clin. Exp. Metastasis 29, 381–395 - PubMed
-
- Gupta G. P., Massagué J. (2006) Cancer metastasis: building a framework. Cell 127, 679–695 - PubMed
-
- Webb B. A., Chimenti M., Jacobson M. P., Barber D. L. (2011) Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 11, 671–677 - PubMed
-
- Martínez-Zaguilán R., Seftor E. A., Seftor R. E., Chu Y. W., Gillies R. J., Hendrix M. J. (1996) Acidic pH enhances the invasive behavior of human melanoma cells. Clin. Exp. Metastasis 14, 176–186 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

