Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data
- PMID: 25505238
- PMCID: PMC4296196
- DOI: 10.1093/jnci/dju366
Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data
Abstract
Background: The results of the European Randomized Study of Screening for Prostate Cancer (ERSPC) trial showed a statistically significant 29% prostate cancer mortality reduction for the men screened in the intervention arm and a 23% negative impact on the life-years gained because of quality of life. However, alternative prostate-specific antigen (PSA) screening strategies for the population may exist, optimizing the effects on mortality reduction, quality of life, overdiagnosis, and costs.
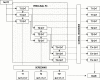
Methods: Based on data of the ERSPC trial, we predicted the numbers of prostate cancers diagnosed, prostate cancer deaths averted, life-years and quality-adjusted life-years (QALY) gained, and cost-effectiveness of 68 screening strategies starting at age 55 years, with a PSA threshold of 3, using microsimulation modeling. The screening strategies varied by age to stop screening and screening interval (one to 14 years or once in a lifetime screens), and therefore number of tests.
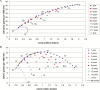
Results: Screening at short intervals of three years or less was more cost-effective than using longer intervals. Screening at ages 55 to 59 years with two-year intervals had an incremental cost-effectiveness ratio of $73000 per QALY gained and was considered optimal. With this strategy, lifetime prostate cancer mortality reduction was predicted as 13%, and 33% of the screen-detected cancers were overdiagnosed. When better quality of life for the post-treatment period could be achieved, an older age of 65 to 72 years for ending screening was obtained.
Conclusion: Prostate cancer screening can be cost-effective when it is limited to two or three screens between ages 55 to 59 years. Screening above age 63 years is less cost-effective because of loss of QALYs because of overdiagnosis.
© The Author 2014. Published by Oxford University Press. All rights reserved. For Permissions, please e-mail: journals.permissions@oup.com.
Figures

Comment in
-
Re: Cost-effectiveness of prostate cancer screening: a simulation study based on ERSPC data.J Natl Cancer Inst. 2015 Apr 16;107(6):djv110. doi: 10.1093/jnci/djv110. Print 2015 Jun. J Natl Cancer Inst. 2015. PMID: 25888716 No abstract available.
-
Response.J Natl Cancer Inst. 2015 Apr 16;107(6):djv111. doi: 10.1093/jnci/djv111. Print 2015 Jun. J Natl Cancer Inst. 2015. PMID: 25888717 No abstract available.
-
Re: Cost-Effectiveness of Prostate Cancer Screening: A Simulation Study Based on ERSPC Data.J Urol. 2015 Jul;194(1):113-4. doi: 10.1016/j.juro.2015.04.064. Epub 2015 Apr 18. J Urol. 2015. PMID: 26088223 No abstract available.
References
-
- Garg V, Gu NY, Borrego ME, et al. A literature review of cost-effectiveness analyses of prostate-specific antigen test in prostate cancer screening. Expert Rev Pharmacoecon Outcomes Res 2013;13(3):327–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

