A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway
- PMID: 25505251
- PMCID: PMC4317025
- DOI: 10.1074/jbc.M114.610733
A gut microbial metabolite of linoleic acid, 10-hydroxy-cis-12-octadecenoic acid, ameliorates intestinal epithelial barrier impairment partially via GPR40-MEK-ERK pathway
Abstract
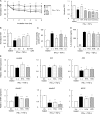
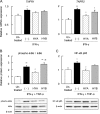
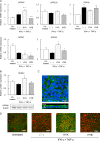
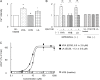
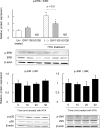
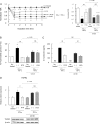
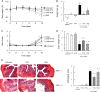
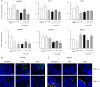
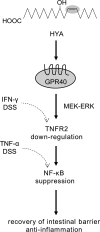
Gut microbial metabolites of polyunsaturated fatty acids have attracted much attention because of their various physiological properties. Dysfunction of tight junction (TJ) in the intestine contributes to the pathogenesis of many disorders such as inflammatory bowel disease. We evaluated the effects of five novel gut microbial metabolites on tumor necrosis factor (TNF)-α-induced barrier impairment in Caco-2 cells and dextran sulfate sodium-induced colitis in mice. 10-Hydroxy-cis-12-octadecenoic acid (HYA), a gut microbial metabolite of linoleic acid, suppressed TNF-α and dextran sulfate sodium-induced changes in the expression of TJ-related molecules, occludin, zonula occludens-1, and myosin light chain kinase. HYA also suppressed the expression of TNF receptor 2 (TNFR2) mRNA and protein expression in Caco-2 cells and colonic tissue. In addition, HYA suppressed the protein expression of TNFR2 in murine intestinal epithelial cells. Furthermore, HYA significantly up-regulated G protein-coupled receptor (GPR) 40 expression in Caco-2 cells. It also induced [Ca(2+)]i responses in HEK293 cells expressing human GPR40 with higher sensitivity than linoleic acid, its metabolic precursor. The barrier-recovering effects of HYA were abrogated by a GPR40 antagonist and MEK inhibitor in Caco-2 cells. Conversely, 10-hydroxyoctadacanoic acid, which is a gut microbial metabolite of oleic acid and lacks a carbon-carbon double bond at Δ12 position, did not show these TJ-restoring activities and down-regulated GPR40 expression. Therefore, HYA modulates TNFR2 expression, at least partially, via the GPR40-MEK-ERK pathway and may be useful in the treatment of TJ-related disorders such as inflammatory bowel disease.
Keywords: Caco-2; Colitis; Epithelial Cell; G Protein-coupled Receptor; Linoleic Acid; Metabolite; Microbiome; Tight Junction; Tumor Necrosis Factor (TNF).
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Gerasimenko J. V., Gryshchenko O., Ferdek P. E., Stapleton E., Hébert T. O., Bychkova S., Peng S., Begg M., Gerasimenko O. V., Petersen O. H. (2013) Ca2+ release-activated Ca2+ channel blockade as a potential tool in antipancreatitis therapy. Proc. Natl. Acad. Sci. U.S.A. 110, 13186–13191 - PMC - PubMed
-
- Kishino S., Takeuchi M., Park S. B., Hirata A., Kitamura N., Kunisawa J., Kiyono H., Iwamoto R., Isobe Y., Arita M., Arai H., Ueda K., Shima J., Takahashi S., Yokozeki K., Shimizu S., Ogawa J. (2013) Polyunsaturated fatty acid saturation by gut lactic acid bacteria affecting host lipid composition. Proc. Natl. Acad. Sci. U.S.A. 110, 17808–17813 - PMC - PubMed
-
- Ogawa J., Kishino S., Ando A., Sugimoto S., Mihara K., Shimizu S. (2005) Production of conjugated fatty acids by lactic acid bacteria. J. Biosci. Bioeng. 100, 355–364 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

