Fancd2 is required for nuclear retention of Foxo3a in hematopoietic stem cell maintenance
- PMID: 25505262
- PMCID: PMC4317007
- DOI: 10.1074/jbc.M114.619536
Fancd2 is required for nuclear retention of Foxo3a in hematopoietic stem cell maintenance
Abstract
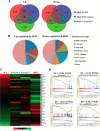
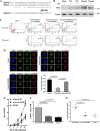
Functional maintenance of hematopoietic stem cells (HSCs) is constantly challenged by stresses like DNA damage and oxidative stress. Here we show that the Fanconi anemia protein Fancd2 and stress transcriptional factor Foxo3a cooperate to prevent HSC exhaustion in mice. Deletion of both Fancd2 and Foxo3a led to an initial expansion followed by a progressive decline of bone marrow stem and progenitor cells. Limiting dilution transplantation and competitive repopulating experiments demonstrated a dramatic reduction of competitive repopulating units and progressive decline in hematopoietic repopulating ability of double-knockout (dKO) HSCs. Analysis of the transcriptome of dKO HSCs revealed perturbation of multiple pathways implicated in HSC exhaustion. Fancd2 deficiency strongly promoted cytoplasmic localization of Foxo3a in HSCs, and re-expression of Fancd2 completely restored nuclear Foxo3a localization. By co-expressing a constitutively active CA-FOXO3a and WT or a nonubiquitinated Fancd2 in dKO bone marrow stem/progenitor cells, we demonstrated that Fancd2 was required for nuclear retention of CA-FOXO3a and for maintaining hematopoietic repopulation of the HSCs. Collectively, these results implicate a functional interaction between the Fanconi anemia DNA repair and FOXO3a pathways in HSC maintenance.
Keywords: DNA Damage; DNA Repair; FOXO; Fancd2; Hematopoietic Stem Cells; Oxidative Stress.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



References
-
- Wilson A., Trumpp A. (2006) Bone-marrow hematopoietic-stem-cell niches. Nat. Rev. Immunol. 6, 93–106 - PubMed
-
- Orford K. W., Scadden D. T. (2008) Deconstructing stem cell self-renewal: genetic insights into cell-cycle regulation. Nat. Rev. Genet. 9, 115–128 - PubMed
-
- Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., Offner S., Dunant C. F., Eshkind L., Bockamp E., Lió P., Macdonald H. R., Trumpp A. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

