Odorant concentration differentiator for intermittent olfactory signals
- PMID: 25505311
- PMCID: PMC6608502
- DOI: 10.1523/JNEUROSCI.2319-14.2014
Odorant concentration differentiator for intermittent olfactory signals
Abstract
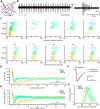
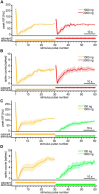
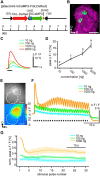
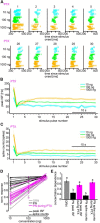
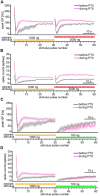
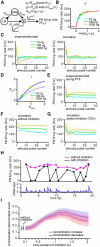
Animals need to discriminate differences in spatiotemporally distributed sensory signals in terms of quality as well as quantity for generating adaptive behavior. Olfactory signals characterized by odor identity and concentration are intermittently distributed in the environment. From these intervals of stimulation, animals process odorant concentration to localize partners or food sources. Although concentration-response characteristics in olfactory neurons have traditionally been investigated using single stimulus pulses, their behavior under intermittent stimulus regimens remains largely elusive. Using the silkmoth (Bombyx mori) pheromone processing system, a simple and behaviorally well-defined model for olfaction, we investigated the neuronal representation of odorant concentration upon intermittent stimulation in the naturally occurring range. To the first stimulus in a series, the responses of antennal lobe (AL) projection neurons (PNs) showed a concentration dependence as previously shown in many olfactory systems. However, PN response amplitudes dynamically changed upon exposure to intermittent stimuli of the same odorant concentration and settled to a constant, largely concentration-independent level. As a result, PN responses emphasized odorant concentration changes rather than encoding absolute concentration in pulse trains of stimuli. Olfactory receptor neurons did not contribute to this response transformation which was due to long-lasting inhibition affecting PNs in the AL. Simulations confirmed that inhibition also provides advantages when stimuli have naturalistic properties. The primary olfactory center thus functions as an odorant concentration differentiator to efficiently detect concentration changes, thereby improving odorant source orientation over a wide concentration range.
Keywords: adaptation; insect; olfaction; sensory intensity.
Copyright © 2014 the authors 0270-6474/14/3416581-13$15.00/0.
Figures

Similar articles
-
Odorant-specific requirements for arrestin function in Drosophila olfaction.J Neurobiol. 2005 Apr;63(1):15-28. doi: 10.1002/neu.20113. J Neurobiol. 2005. PMID: 15627264
-
Representation of a mixture of pheromone and host plant odor by antennal lobe projection neurons of the silkmoth Bombyx mori.J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008 May;194(5):501-15. doi: 10.1007/s00359-008-0325-3. Epub 2008 Apr 4. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2008. PMID: 18389256
-
Postsynaptic odorant concentration dependent inhibition controls temporal properties of spike responses of projection neurons in the moth antennal lobe.PLoS One. 2014 Feb 19;9(2):e89132. doi: 10.1371/journal.pone.0089132. eCollection 2014. PLoS One. 2014. PMID: 24586546 Free PMC article.
-
Olfactory maps and odor images.Curr Opin Neurobiol. 2002 Aug;12(4):387-92. doi: 10.1016/s0959-4388(02)00348-3. Curr Opin Neurobiol. 2002. PMID: 12139985 Review.
-
The olfactory bulb: coding and processing of odor molecule information.Science. 1999 Oct 22;286(5440):711-5. doi: 10.1126/science.286.5440.711. Science. 1999. PMID: 10531048 Review.
Cited by
-
Two types of local interneurons are distinguished by morphology, intrinsic membrane properties, and functional connectivity in the moth antennal lobe.J Neurophysiol. 2015 Nov;114(5):3002-13. doi: 10.1152/jn.00050.2015. Epub 2015 Sep 16. J Neurophysiol. 2015. PMID: 26378200 Free PMC article.
-
Temporal characteristics of turbulent flow and moth orientation behaviour patterns with fluent simulation and moth-based moving model simulation.Heliyon. 2024 Aug 28;10(17):e37004. doi: 10.1016/j.heliyon.2024.e37004. eCollection 2024 Sep 15. Heliyon. 2024. PMID: 39281631 Free PMC article.
-
Simultaneous recording of spikes and calcium signals in odor-evoked responses of Drosophila antennal neurons.bioRxiv [Preprint]. 2025 Jun 29:2025.06.27.662059. doi: 10.1101/2025.06.27.662059. bioRxiv. 2025. PMID: 40666897 Free PMC article. Preprint.
-
Targeted disruption of a single sex pheromone receptor gene completely abolishes in vivo pheromone response in the silkmoth.Sci Rep. 2015 Jun 5;5:11001. doi: 10.1038/srep11001. Sci Rep. 2015. PMID: 26047360 Free PMC article.
-
Insulin restores retinal ganglion cell functional connectivity and promotes visual recovery in glaucoma.Sci Adv. 2024 Aug 9;10(32):eadl5722. doi: 10.1126/sciadv.adl5722. Epub 2024 Aug 7. Sci Adv. 2024. PMID: 39110798 Free PMC article.
References
-
- Baker TC, Roelofs WL. Initiation and termination of oriental fruit moth male response to pheromone concentrations in the field. Environ Entomol. 1981;10:211–218.
-
- Baker TC, Willis MA, Haynes KF, Phelan PL. A pulsed cloud of sex-pheromone elicits upwind flight in male moths. Physiol Entomol. 1985;10:257–265. doi: 10.1111/j.1365-3032.1985.tb00045.x. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
