Live imaging of endogenous PSD-95 using ENABLED: a conditional strategy to fluorescently label endogenous proteins
- PMID: 25505322
- PMCID: PMC4261096
- DOI: 10.1523/JNEUROSCI.3888-14.2014
Live imaging of endogenous PSD-95 using ENABLED: a conditional strategy to fluorescently label endogenous proteins
Abstract
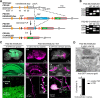
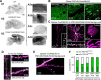
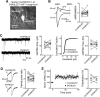
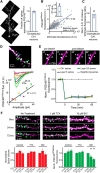
Stoichiometric labeling of endogenous synaptic proteins for high-contrast live-cell imaging in brain tissue remains challenging. Here, we describe a conditional mouse genetic strategy termed endogenous labeling via exon duplication (ENABLED), which can be used to fluorescently label endogenous proteins with near ideal properties in all neurons, a sparse subset of neurons, or specific neuronal subtypes. We used this method to label the postsynaptic density protein PSD-95 with mVenus without overexpression side effects. We demonstrated that mVenus-tagged PSD-95 is functionally equivalent to wild-type PSD-95 and that PSD-95 is present in nearly all dendritic spines in CA1 neurons. Within spines, while PSD-95 exhibited low mobility under basal conditions, its levels could be regulated by chronic changes in neuronal activity. Notably, labeled PSD-95 also allowed us to visualize and unambiguously examine otherwise-unidentifiable excitatory shaft synapses in aspiny neurons, such as parvalbumin-positive interneurons and dopaminergic neurons. Our results demonstrate that the ENABLED strategy provides a valuable new approach to study the dynamics of endogenous synaptic proteins in vivo.
Keywords: PSD-95; cell-type-specific labeling; conditional knock-in; live imaging; protein labeling; sparse labeling.
Copyright © 2014 the authors 0270-6474/14/3416698-15$15.00/0.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
