Human scalp potentials reflect a mixture of decision-related signals during perceptual choices
- PMID: 25505339
- PMCID: PMC4261107
- DOI: 10.1523/JNEUROSCI.3012-14.2014
Human scalp potentials reflect a mixture of decision-related signals during perceptual choices
Abstract
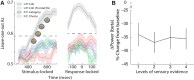
Single-unit animal studies have consistently reported decision-related activity mirroring a process of temporal accumulation of sensory evidence to a fixed internal decision boundary. To date, our understanding of how response patterns seen in single-unit data manifest themselves at the macroscopic level of brain activity obtained from human neuroimaging data remains limited. Here, we use single-trial analysis of human electroencephalography data to show that population responses on the scalp can capture choice-predictive activity that builds up gradually over time with a rate proportional to the amount of sensory evidence, consistent with the properties of a drift-diffusion-like process as characterized by computational modeling. Interestingly, at time of choice, scalp potentials continue to appear parametrically modulated by the amount of sensory evidence rather than converging to a fixed decision boundary as predicted by our model. We show that trial-to-trial fluctuations in these response-locked signals exert independent leverage on behavior compared with the rate of evidence accumulation earlier in the trial. These results suggest that in addition to accumulator signals, population responses on the scalp reflect the influence of other decision-related signals that continue to covary with the amount of evidence at time of choice.
Keywords: EEG; confidence; diffusion model; evidence accumulation; perceptual decision making; single-trial.
Copyright © 2014 Philiastides et al.
Figures





References
-
- Dakin SC, Hess RF, Ledgeway T, Achtman RL. What causes non-monotonic tuning of fMRI response to noisy images? Curr Biol. 2002;12:R476–R477. author reply R478. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
