Fetal autonomic brain age scores, segmented heart rate variability analysis, and traditional short term variability
- PMID: 25505399
- PMCID: PMC4243554
- DOI: 10.3389/fnhum.2014.00948
Fetal autonomic brain age scores, segmented heart rate variability analysis, and traditional short term variability
Abstract
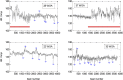
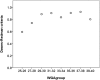
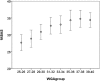
Disturbances of fetal autonomic brain development can be evaluated from fetal heart rate patterns (HRP) reflecting the activity of the autonomic nervous system. Although HRP analysis from cardiotocographic (CTG) recordings is established for fetal surveillance, temporal resolution is low. Fetal magnetocardiography (MCG), however, provides stable continuous recordings at a higher temporal resolution combined with a more precise heart rate variability (HRV) analysis. A direct comparison of CTG and MCG based HRV analysis is pending. The aims of the present study are: (i) to compare the fetal maturation age predicting value of the MCG based fetal Autonomic Brain Age Score (fABAS) approach with that of CTG based Dawes-Redman methodology; and (ii) to elaborate fABAS methodology by segmentation according to fetal behavioral states and HRP. We investigated MCG recordings from 418 normal fetuses, aged between 21 and 40 weeks of gestation. In linear regression models we obtained an age predicting value of CTG compatible short term variability (STV) of R (2) = 0.200 (coefficient of determination) in contrast to MCG/fABAS related multivariate models with R (2) = 0.648 in 30 min recordings, R (2) = 0.610 in active sleep segments of 10 min, and R (2) = 0.626 in quiet sleep segments of 10 min. Additionally segmented analysis under particular exclusion of accelerations (AC) and decelerations (DC) in quiet sleep resulted in a novel multivariate model with R (2) = 0.706. According to our results, fMCG based fABAS may provide a promising tool for the estimation of fetal autonomic brain age. Beside other traditional and novel HRV indices as possible indicators of developmental disturbances, the establishment of a fABAS score normogram may represent a specific reference. The present results are intended to contribute to further exploration and validation using independent data sets and multicenter research structures.
Keywords: cardiotocography; fetal autonomic brain age; magnetocardiography; prenatal diagnosis.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources

