Sensing deep extreme environments: the receptor cell types, brain centers, and multi-layer neural packaging of hydrothermal vent endemic worms
- PMID: 25505488
- PMCID: PMC4261566
- DOI: 10.1186/s12983-014-0082-9
Sensing deep extreme environments: the receptor cell types, brain centers, and multi-layer neural packaging of hydrothermal vent endemic worms
Abstract
Introduction: Deep-sea alvinellid worm species endemic to hydrothermal vents, such as Alvinella and Paralvinella, are considered to be among the most thermotolerant animals known with their adaptability to toxic heavy metals, and tolerance of highly reductive and oxidative stressful environments. Despite the number of recent studies focused on their overall transcriptomic, proteomic, and metabolic stabilities, little is known regarding their sensory receptor cells and electrically active neuro-processing centers, and how these can tolerate and function in such harsh conditions.
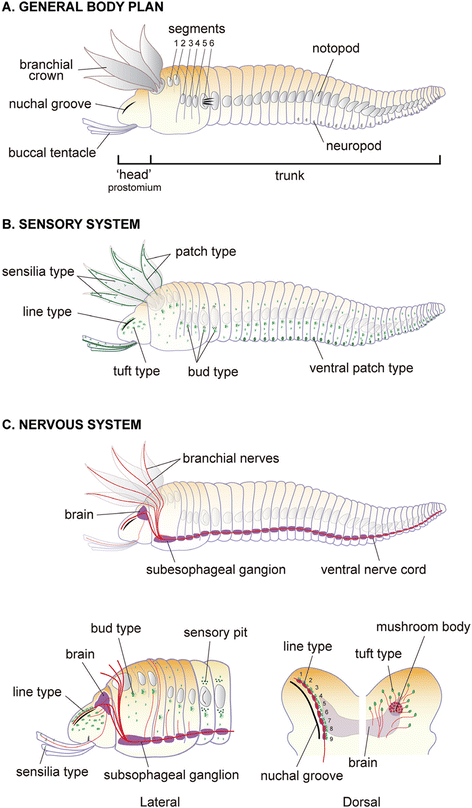
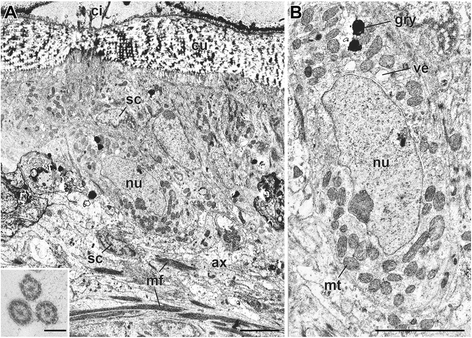
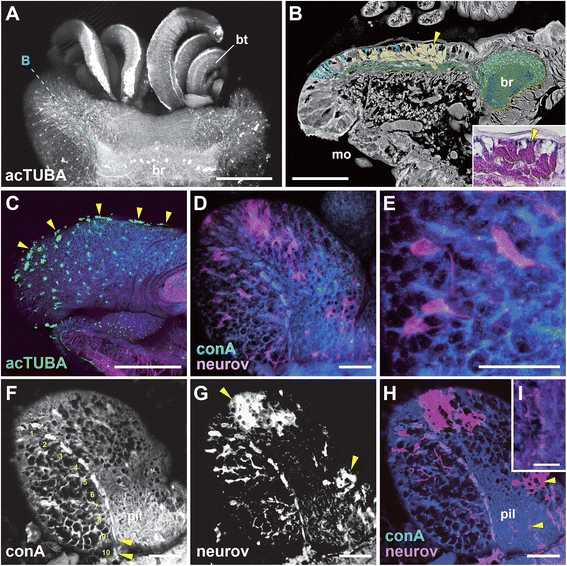
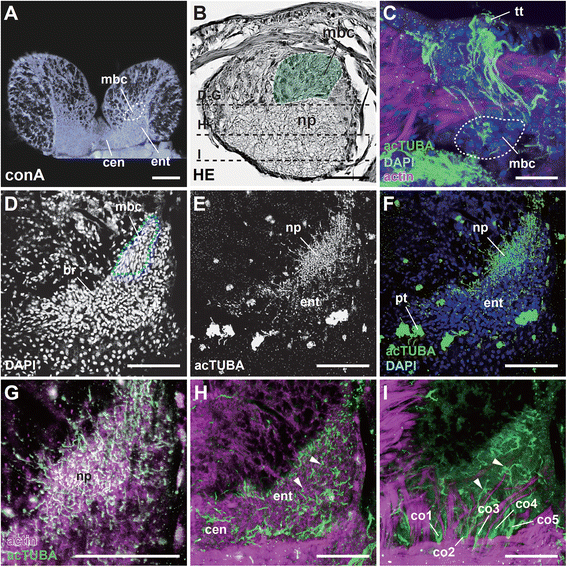
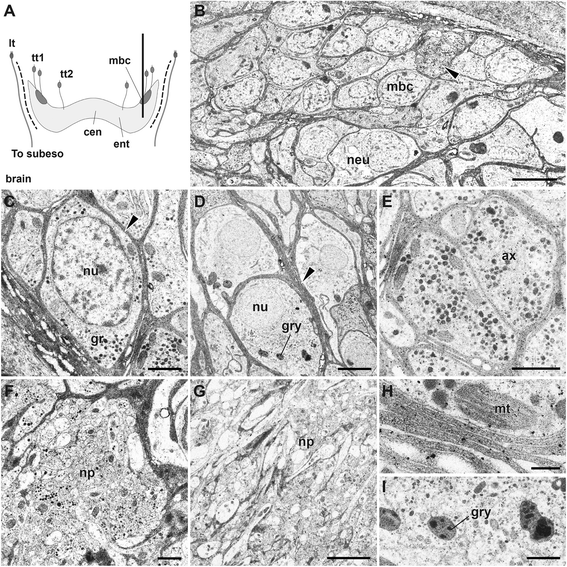
Results: We examined the extra- and intracellular organizations of the epidermal ciliated sensory cells and their higher centers in the central nervous system through immunocytochemical, ultrastructural, and neurotracing analyses. We observed that these cells were rich in mitochondria and possessed many electron-dense granules, and identified specialized glial cells and serial myelin-like repeats in the head sensory systems of Paralvinella hessleri. Additionally, we identified the major epidermal sensory pathways, in which a pair of distinct mushroom bodies-like or small interneuron clusters was observed. These sensory learning and memory systems are commonly found in insects and annelids, but the alvinellid inputs are unlikely derived from the sensory ciliary cells of the dorsal head regions.
Conclusions: Our evidence provides insight into the cellular and system-wide adaptive structure used to sense, process, and combat the deep-sea hydrothermal vent environment. The alvinellid sensory cells exhibit characteristics of annelid ciliary types, and among the most unique features were the head sensory inputs and structure of the neural cell bodies of the brain, which were surrounded by multiple membranes. We speculated that such enhanced protection is required for the production of normal electrical signals, and to avoid the breakdown of the membrane surrounding metabolically fragile neurons from oxidative stress. Such pivotal acquisition is not broadly found in the all body parts, suggesting the head sensory inputs are specific, and these heterogenetic protection mechanisms may be present in alvinellid worms.
Keywords: Annelids; Brain; Deep-sea; Evolution; Glia; Nervous system; Sensory cells.
Figures







Similar articles
-
Highly sensitive avoidance plays a key role in sensory adaptation to deep-sea hydrothermal vent environments.PLoS One. 2018 Jan 3;13(1):e0189902. doi: 10.1371/journal.pone.0189902. eCollection 2018. PLoS One. 2018. PMID: 29298328 Free PMC article.
-
Dual Cellular Supporters: Multi-Layer Glial Wrapping and the Penetrative Matrix Specialized in Deep-Sea Hydrothermal Vent Endemic Scale-Worms.Biol Bull. 2015 Jun;228(3):217-26. doi: 10.1086/BBLv228n3p217. Biol Bull. 2015. PMID: 26124448
-
Extracellular Hemoglobins of Hydrothermal Vent Annelids: Structural and Functional Characteristics in Three Alvinellid Species.Biol Bull. 1990 Dec;179(3):366-373. doi: 10.2307/1542329. Biol Bull. 1990. PMID: 29314955
-
Deep-sea hydrothermal vents: potential hot spots for natural products discovery?J Nat Prod. 2010 Mar 26;73(3):489-99. doi: 10.1021/np900662k. J Nat Prod. 2010. PMID: 20099811 Review.
-
Metazoans in extreme environments: adaptations of hydrothermal vent and hydrocarbon seep fauna.Gravit Space Biol Bull. 2000 Jun;13(2):13-23. Gravit Space Biol Bull. 2000. PMID: 11543277 Review.
Cited by
-
Toward an MRI-Based Mesoscale Connectome of the Squid Brain.iScience. 2020 Jan 24;23(1):100816. doi: 10.1016/j.isci.2019.100816. Epub 2020 Jan 2. iScience. 2020. PMID: 31972515 Free PMC article.
-
The neuroanatomy of the siboglinid Riftia pachyptila highlights sedentarian annelid nervous system evolution.PLoS One. 2018 Dec 13;13(12):e0198271. doi: 10.1371/journal.pone.0198271. eCollection 2018. PLoS One. 2018. PMID: 30543637 Free PMC article.
-
Post-translational modifications are enriched within protein functional groups important to bacterial adaptation within a deep-sea hydrothermal vent environment.Microbiome. 2016 Sep 6;4(1):49. doi: 10.1186/s40168-016-0194-x. Microbiome. 2016. PMID: 27600525 Free PMC article.
-
Ultrastructure and functional morphology of the appendages in the reef-building sedentary polychaete Sabellaria alveolata (Annelida, Sedentaria, Sabellida).BMC Zool. 2021 Mar 9;6(1):5. doi: 10.1186/s40850-021-00068-8. BMC Zool. 2021. PMID: 37170289 Free PMC article.
-
The role of the home environment in neurocognitive development of children living in extreme poverty and with frequent illnesses: a cross-sectional study.Wellcome Open Res. 2018 Dec 3;3:152. doi: 10.12688/wellcomeopenres.14702.1. eCollection 2018. Wellcome Open Res. 2018. PMID: 30687794 Free PMC article.
References
-
- Desbruyeres D, Laubier L. Systematics, phylogeny, ecology and distribution of the Alvinellidae (Polychaeta) from deep-sea hydrothermal vents. Ophelia. 1991;5:31–45.
-
- Desbruyeres D, Chevaldonne P, Alayse AM, Jollivet D, Lallier FH, Jouin-Toulmond C, Zal F, Sarradin PM, Cosson R, Caprais JC, Arndt C, O’Brien J, Guezennec J, Hourdez S, Riso R, Gaill F, Laubier L, Toulmond A. Biology and ecology of the “Pompeii worm” (Alvinella pompejana Desbruyeres and Laubier), a normal dweller of an extreme deep-sea environment: a synthesis of current knowledge and recent developments. Deep-Sea Res Part I. 1998;45:383–422. doi: 10.1016/S0967-0645(97)00083-0. - DOI
-
- Chevaldonne P, Fisher CR, Childress JJ, Desbruyeres D, Jollivet D, Zal F, Toulmond A. Thermotolerance and the “pompeii worms”. Mar Ecol Prog Ser. 2000;208:293–295. doi: 10.3354/meps208293. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

