Glucuronidation of drugs in humanized UDP-glucuronosyltransferase 1 mice: Similarity with glucuronidation in human liver microsomes
- PMID: 25505556
- PMCID: PMC4184567
- DOI: 10.1002/prp2.2
Glucuronidation of drugs in humanized UDP-glucuronosyltransferase 1 mice: Similarity with glucuronidation in human liver microsomes
Abstract
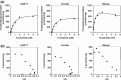
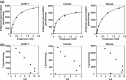
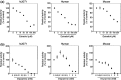
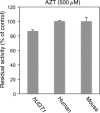
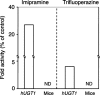
Uridine 5'-diphosphate-glucuronosyltransferases (UGTs) are phase II drug-metabolizing enzymes that catalyze glucuronidation of various endogenous and exogenous substrates. Among 19 functional human UGTs, UGT1A family enzymes largely contribute to the metabolism of clinically used drugs. While the UGT1A locus is conserved in mammals such as humans, mice, and rats, species differences in drug glucuronidation have been reported. Recently, humanized UGT1 mice in which the original Ugt1 locus was disrupted and replaced with the human UGT1 locus (hUGT1 mice) have been developed. To evaluate the usefulness of hUGT1 mice to predict human glucuronidation of drugs, UGT activities, and inhibitory effects on UGTs were examined in liver microsomes of hUGT1 mice as well as in those of wild-type mice and humans. Furosemide acyl-glucuronidation was sigmoidal and best fitted to the Hill equation in hUGT1 mice and human liver microsomes, while it was fitted to the substrate inhibition equation in mouse liver microsomes. Kinetic parameters of furosemide glucuronidation were very similar between hUGT1 mice and human liver microsomes. The kinetics of S-naproxen acyl-glucuronidation and inhibitory effects of compounds on furosemide glucuronidation in hUGT1 liver microsomes were also slightly, but similar to those in human liver microsomes, rather than in wild-type mice. While wild-type mice lack imipramine and trifluoperazine N-glucuronidation potential, hUGT1 mice showed comparable N-glucuronidation activity to that of humans. Our data indicate that hUGT1 mice are promising tools to predict not only in vivo human drug glucuronidation but also potential drug-drug interactions.
Keywords: Humanized animal model; UDP-glucuronosyltransferase; UGT; drug metabolism; species difference.
Figures

Similar articles
-
Glucuronidation of drugs and drug-induced toxicity in humanized UDP-glucuronosyltransferase 1 mice.Drug Metab Dispos. 2014 Jul;42(7):1146-52. doi: 10.1124/dmd.114.057083. Epub 2014 Apr 24. Drug Metab Dispos. 2014. PMID: 24764149 Free PMC article.
-
Expression of UDP-Glucuronosyltransferase 1 (UGT1) and Glucuronidation Activity toward Endogenous Substances in Humanized UGT1 Mouse Brain.Drug Metab Dispos. 2015 Jul;43(7):1071-6. doi: 10.1124/dmd.115.063719. Epub 2015 May 7. Drug Metab Dispos. 2015. PMID: 25953521 Free PMC article.
-
Species differences in drug glucuronidation: Humanized UDP-glucuronosyltransferase 1 mice and their application for predicting drug glucuronidation and drug-induced toxicity in humans.Drug Metab Pharmacokinet. 2018 Feb;33(1):9-16. doi: 10.1016/j.dmpk.2017.10.002. Epub 2017 Oct 7. Drug Metab Pharmacokinet. 2018. PMID: 29079228 Free PMC article. Review.
-
Nicotine regulates the expression of UDP-glucuronosyltransferase (UGT) in humanized UGT1 mouse brain.Drug Metab Pharmacokinet. 2015 Aug;30(4):269-75. doi: 10.1016/j.dmpk.2015.04.004. Epub 2015 May 7. Drug Metab Pharmacokinet. 2015. PMID: 26210671 Free PMC article.
-
Structure and Protein-Protein Interactions of Human UDP-Glucuronosyltransferases.Front Pharmacol. 2016 Oct 24;7:388. doi: 10.3389/fphar.2016.00388. eCollection 2016. Front Pharmacol. 2016. PMID: 27822186 Free PMC article. Review.
Cited by
-
Glucuronidation of drugs and drug-induced toxicity in humanized UDP-glucuronosyltransferase 1 mice.Drug Metab Dispos. 2014 Jul;42(7):1146-52. doi: 10.1124/dmd.114.057083. Epub 2014 Apr 24. Drug Metab Dispos. 2014. PMID: 24764149 Free PMC article.
-
Prediction of drug-induced liver injury using keratinocytes.J Appl Toxicol. 2017 Jul;37(7):863-872. doi: 10.1002/jat.3435. Epub 2017 Jan 31. J Appl Toxicol. 2017. PMID: 28138970 Free PMC article.
-
Salicylic Acid Is Required for Broad-Spectrum Disease Resistance in Rice.Int J Mol Sci. 2022 Jan 25;23(3):1354. doi: 10.3390/ijms23031354. Int J Mol Sci. 2022. PMID: 35163275 Free PMC article.
-
Expression of UDP-Glucuronosyltransferase 1 (UGT1) and Glucuronidation Activity toward Endogenous Substances in Humanized UGT1 Mouse Brain.Drug Metab Dispos. 2015 Jul;43(7):1071-6. doi: 10.1124/dmd.115.063719. Epub 2015 May 7. Drug Metab Dispos. 2015. PMID: 25953521 Free PMC article.
-
Molecular Pathways: GLI1-Induced Drug Glucuronidation in Resistant Cancer Cells.Clin Cancer Res. 2015 May 15;21(10):2207-10. doi: 10.1158/1078-0432.CCR-14-1370. Epub 2015 Mar 25. Clin Cancer Res. 2015. PMID: 25810373 Free PMC article.
References
-
- Al-Zoughool M, Talaska G. High-performance liquid chromatography method for determination of N-glucuronidation of 4-aminobiphenyl by mouse, rat, and human liver microsomes. Anal Biochem. 2005;340:352–358. - PubMed
-
- Bowalgaha K, Elliot DJ, Mackenzie PI, Knights KM, Swedmark S, Miners JO. S-Naproxen and desmethylnaproxen glucuronidation by human liver microsomes and recombinant human UDP-glucuronosyltransferases (UGT): role of UGT2B7 in the elimination of naproxen. Br J Clin Pharmacol. 2005;60:423–433. - PMC - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Chen S, Beaton D, Nguyen N, Senekeo-Effenberger K, Brace-Sinnokrak E, Argikar U, et al. Tissue-specific, inducible, and hormonal control of the human UDP-glucuronosyltransferase-1 (UGT1) locus. J Biol Chem. 2005;280:37547–37557. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

