XG-102 administered to healthy male volunteers as a single intravenous infusion: a randomized, double-blind, placebo-controlled, dose-escalating study
- PMID: 25505576
- PMCID: PMC4186400
- DOI: 10.1002/prp2.20
XG-102 administered to healthy male volunteers as a single intravenous infusion: a randomized, double-blind, placebo-controlled, dose-escalating study
Abstract
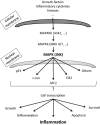
The aim of the study is to evaluate the safety, tolerability and pharmacokinetics (PK) of the JNK inhibitor XG-102 in a randomized, double blind, placebo controlled, sequential ascending dose parallel group Phase 1 Study. Three groups of male subjects received as randomly assigned ascending single XG-102 doses (10, 40, and 80 μg/kg; 6 subjects per dose) or placebo (2 subjects per dose) as an intravenous (IV) infusion over 60 min. Safety and tolerability were assessed by physical examination, vital signs, electrocardiography, eye examination, clinical laboratory tests and adverse events (AEs). PK was analyzed using noncompartmental methods. All reported AEs were mild to moderate and neither their number nor their distribution by System Organ Class suggest a dose relationship. Only headache and fatigue were considered probably or possibly study drug related. Headache frequency was similar for active and placebo, consequently this was not considered to be drug related but probably to study conditions. The other examinations did not show clinically relevant deviations or trends suggesting a XG-102 relationship. Geometric mean half-life was similar among doses, ranging from 0.36 to 0.65 h. Geometric mean XG-102 AUC0-last increased more than linearly with dose, 90% confidence intervals (CIs) did not overlap for the two highest doses. Geometric mean dose normalized C max values suggest a more than linear increase with dose but 90% CIs overlap. It may be concluded that XG-102 single IV doses of 10-80 μg/kg administered over 1 h to healthy male subjects were safe and well tolerated.
Keywords: Clinical study; PK; XG-102; healthy volunteers; infusion; intravenous; phase I; safety; tolerability.
Figures
Similar articles
-
Pharmacokinetics, pharmacodynamics, and tolerability of the dipeptidyl peptidase IV inhibitor LC15-0444 in healthy Korean men: a dose-block-randomized, double-blind, placebo-controlled, ascending single-dose, Phase I study.Clin Ther. 2008 Oct;30(10):1817-30. doi: 10.1016/j.clinthera.2008.10.013. Clin Ther. 2008. PMID: 19014837 Clinical Trial.
-
Pharmacokinetics, safety and tolerability of intravenous ferric carboxymaltose: a dose-escalation study in volunteers with mild iron-deficiency anaemia.Arzneimittelforschung. 2010;60(6a):362-72. doi: 10.1055/s-0031-1296301. Arzneimittelforschung. 2010. PMID: 20648928 Clinical Trial.
-
Clinical pharmacology of DP-b99 in healthy volunteers: first administration to humans.Br J Clin Pharmacol. 2005 Jul;60(1):7-16. doi: 10.1111/j.1365-2125.2005.02378.x. Br J Clin Pharmacol. 2005. PMID: 15963088 Free PMC article. Clinical Trial.
-
Single-dose bioavailability of levetiracetam intravenous infusion relative to oral tablets and multiple-dose pharmacokinetics and tolerability of levetiracetam intravenous infusion compared with placebo in healthy subjects.Clin Ther. 2006 May;28(5):734-44. doi: 10.1016/j.clinthera.2006.05.004. Clin Ther. 2006. PMID: 16861095 Clinical Trial.
-
Clevidipine: a review of its use in the management of acute hypertension.Am J Cardiovasc Drugs. 2009;9(2):117-34. doi: 10.2165/00129784-200909020-00006. Am J Cardiovasc Drugs. 2009. PMID: 19331440 Review.
Cited by
-
MAPK Pathways in Ocular Pathophysiology: Potential Therapeutic Drugs and Challenges.Cells. 2023 Feb 14;12(4):617. doi: 10.3390/cells12040617. Cells. 2023. PMID: 36831285 Free PMC article. Review.
-
JNK Regulation of Depression and Anxiety.Brain Plast. 2018 Aug 10;3(2):145-155. doi: 10.3233/BPL-170062. Brain Plast. 2018. PMID: 30151339 Free PMC article. Review.
-
Targeting a key disulfide linkage to regulate RIG-I condensation and cytosolic RNA-sensing.Nat Cell Biol. 2025 May;27(5):817-834. doi: 10.1038/s41556-025-01646-5. Epub 2025 Apr 14. Nat Cell Biol. 2025. PMID: 40229436
-
Cell-Penetrating Peptides Enhance the Activity of Human Fibroblast Growth Factor 2 by Prolonging the Retention Time: A New Vision for Drug-Delivery Systems.Int J Mol Sci. 2020 Jan 10;21(2):442. doi: 10.3390/ijms21020442. Int J Mol Sci. 2020. PMID: 32284513 Free PMC article.
-
c-Jun N-Terminal Kinases (JNKs) in Myocardial and Cerebral Ischemia/Reperfusion Injury.Front Pharmacol. 2018 Jul 5;9:715. doi: 10.3389/fphar.2018.00715. eCollection 2018. Front Pharmacol. 2018. PMID: 30026697 Free PMC article. Review.
References
-
- Antoniou X, Falconi M, Di Marino D, Borsello T. JNK3 as a therapeutic target for neurodegenerative diseases. J Alzheimers Dis. 2011;24:633–642. - PubMed
-
- Benakis C, Bonny C, Hirt L. JNK inhibition and inflammation after cerebral ischemia. Brain Behav Immun. 2010;24:800–811. - PubMed
-
- Bessero AC, Chiodini F, Rungger-Brändle E, Bonny C, Clarke PG. Role of the c-Jun N-terminal kinase pathway in retinal excitotoxicity, and neuroprotection by its inhibition. J Neurochem. 2010;113:1307–1318. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

