Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle
- PMID: 25505577
- PMCID: PMC4186404
- DOI: 10.1002/prp2.28
Database search of spontaneous reports and pharmacological investigations on the sulfonylureas and glinides-induced atrophy in skeletal muscle
Abstract
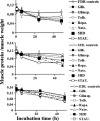
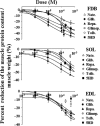
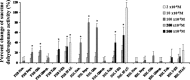
The ATP-sensitive K(+) (KATP) channel is an emerging pathway in the skeletal muscle atrophy which is a comorbidity condition in diabetes. The "in vitro" effects of the sulfonylureas and glinides were evaluated on the protein content/muscle weight, fibers viability, mitochondrial succinic dehydrogenases (SDH) activity, and channel currents in oxidative soleus (SOL), glycolitic/oxidative flexor digitorum brevis (FDB), and glycolitic extensor digitorum longus (EDL) muscle fibers of mice using biochemical and cell-counting Kit-8 assay, image analysis, and patch-clamp techniques. The sulfonylureas were: tolbutamide, glibenclamide, and glimepiride; the glinides were: repaglinide and nateglinide. Food and Drug Administration-Adverse Effects Reporting System (FDA-AERS) database searching of atrophy-related signals associated with the use of these drugs in humans has been performed. The drugs after 24 h of incubation time reduced the protein content/muscle weight and fibers viability more effectively in FDB and SOL than in the EDL. The order of efficacy of the drugs in reducing the protein content in FDB was: repaglinide (EC50 = 5.21 × 10(-6)) ≥ glibenclamide(EC50 = 8.84 × 10(-6)) > glimepiride(EC50 = 2.93 × 10(-5)) > tolbutamide(EC50 = 1.07 × 10(-4)) > nateglinide(EC50 = 1.61 × 10(-4)) and it was: repaglinide(7.15 × 10(-5)) ≥ glibenclamide(EC50 = 9.10 × 10(-5)) > nateglinide(EC50 = 1.80 × 10(-4)) ≥ tolbutamide(EC50 = 2.19 × 10(-4)) > glimepiride(EC50=-) in SOL. The drug-induced atrophy can be explained by the KATP channel block and by the enhancement of the mitochondrial SDH activity. In an 8-month period, muscle atrophy was found in 0.27% of the glibenclamide reports in humans and in 0.022% of the other not sulfonylureas and glinides drugs. No reports of atrophy were found for the other sulfonylureas and glinides in the FDA-AERS. Glibenclamide induces atrophy in animal experiments and in human patients. Glimepiride shows less potential for inducing atrophy.
Keywords: Atrophy; oral antidiabetic drugs; pharmacovigilance; skeletal muscle.
Figures




Similar articles
-
Pathophysiological Consequences of KATP Channel Overactivity and Pharmacological Response to Glibenclamide in Skeletal Muscle of a Murine Model of Cantù Syndrome.Front Pharmacol. 2020 Nov 30;11:604885. doi: 10.3389/fphar.2020.604885. eCollection 2020. Front Pharmacol. 2020. PMID: 33329006 Free PMC article.
-
The KATP channel is a molecular sensor of atrophy in skeletal muscle.J Physiol. 2010 Mar 1;588(Pt 5):773-84. doi: 10.1113/jphysiol.2009.185835. Epub 2010 Jan 11. J Physiol. 2010. PMID: 20064856 Free PMC article.
-
Dual response of the KATP channels to staurosporine: a novel role of SUR2B, SUR1 and Kir6.2 subunits in the regulation of the atrophy in different skeletal muscle phenotypes.Biochem Pharmacol. 2014 Sep 15;91(2):266-75. doi: 10.1016/j.bcp.2014.06.023. Epub 2014 Jul 3. Biochem Pharmacol. 2014. PMID: 24998494
-
Characterization of the molecular mode of action of the sulfonylurea, glimepiride, at adipocytes.Horm Metab Res. 1996 Sep;28(9):469-87. doi: 10.1055/s-2007-979839. Horm Metab Res. 1996. PMID: 8911985 Review.
-
Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II.Curr Diabetes Rev. 2014;10(4):231-7. doi: 10.2174/1573399810666140918121022. Curr Diabetes Rev. 2014. PMID: 25245021 Review.
Cited by
-
Effects of anti-diabetic drugs on sarcopenia: Best treatment options for elderly patients with type 2 diabetes mellitus and sarcopenia.World J Clin Cases. 2021 Nov 26;9(33):10064-10074. doi: 10.12998/wjcc.v9.i33.10064. World J Clin Cases. 2021. PMID: 34904076 Free PMC article. Review.
-
Zoledronic Acid Blocks Overactive Kir6.1/SUR2-Dependent KATP Channels in Skeletal Muscle and Osteoblasts in a Murine Model of Cantú Syndrome.Cells. 2023 Mar 17;12(6):928. doi: 10.3390/cells12060928. Cells. 2023. PMID: 36980269 Free PMC article.
-
Drug-related sarcopenia as a secondary sarcopenia.Geriatr Gerontol Int. 2024 Feb;24(2):195-203. doi: 10.1111/ggi.14770. Epub 2023 Dec 29. Geriatr Gerontol Int. 2024. PMID: 38158766 Free PMC article. Review.
-
Imaging of Sarcopenia in Type 2 Diabetes Mellitus.Clin Interv Aging. 2024 Jan 26;19:141-151. doi: 10.2147/CIA.S443572. eCollection 2024. Clin Interv Aging. 2024. PMID: 38292460 Free PMC article. Review.
-
Bisphosphonates Targeting Ion Channels and Musculoskeletal Effects.Front Pharmacol. 2022 Mar 15;13:837534. doi: 10.3389/fphar.2022.837534. eCollection 2022. Front Pharmacol. 2022. PMID: 35370739 Free PMC article. Review.
References
-
- Arnoux JB, de Lonlay P, Ribeiro MJ, Hussain K, Blankenstein O, Mohnike K, et al. Congenital hyperinsulinism. Early Hum Dev. 2010;86:287–294. - PubMed
-
- Ashcroft FM. New uses for old drugs: neonatal diabetes and sulphonylureas. Cell Metab. 2010;11:179–181. - PubMed
-
- Babenko AP, Gonzalez G, Bryan J. Pharmaco-topology of sulfonylurea receptors. Separate domains of the regulatory subunits of K(ATP) channel isoforms are required for selective interaction with K(+) channel openers. J Biol Chem. 2000;275:717–720. - PubMed
-
- De la Cadena SG, Hernández-Fonseca K, Camacho-Arroyo I, Massieu L. Glucose deprivation induces reticulum stress by the PERK pathway and caspase-7- and calpain-mediated caspase-12 activation. Apoptosis. 2013 doi: 10.1007/s10495-013-0930-7. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

