Intestinal absorption of the antiepileptic drug substance vigabatrin is altered by infant formula in vitro and in vivo
- PMID: 25505585
- PMCID: PMC4184708
- DOI: 10.1002/prp2.36
Intestinal absorption of the antiepileptic drug substance vigabatrin is altered by infant formula in vitro and in vivo
Abstract
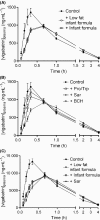
Vigabatrin is an antiepileptic drug substance mainly used in pediatric treatment of infantile spasms. The main source of nutrition for infants is breast milk and/or infant formula. Our hypothesis was that infant formula may affect the intestinal absorption of vigabatrin. The aim was therefore to investigate the potential effect of coadministration of infant formula with vigabatrin on the oral absorption in vitro and in vivo. The effect of vigabatrin given with an infant formula on the oral uptake and transepithelial transport was investigated in vitro in Caco-2 cells. In vivo effects of infant formula and selected amino acids on the pharmacokinetic profile of vigabatrin was investigated after oral coadministration to male Sprague-Dawley rats using acetaminophen as a marker for gastric emptying. The presence of infant formula significantly reduced the uptake rate and permeability of vigabatrin in Caco-2 cells. Oral coadministration of vigabatrin and infant formula significantly reduced C max and prolonged t max of vigabatrin absorption. Ligands for the proton-coupled amino acid transporter PAT1, sarcosine, and proline/l-tryptophan had similar effects on the pharmacokinetic profile of vigabatrin. The infant formula decreased the rate of gastric emptying. Here we provide experimental evidence for an in vivo role of PAT1 in the intestinal absorption of vigabatrin. The effect of infant formula on the oral absorption of vigabatrin was found to be due to delayed gastric emptying, however, it seems reasonable that infant formula may also directly affect the intestinal absorption rate of vigabatrin possibly via PAT1.
Keywords: Caco-2 cells; LYAAT1; PAT1; SLC36A1; Sprague–Dawley rats; Vigabatrin; drug absorption; infant formula; pharmacokinetic.
Figures



Similar articles
-
Intestinal absorption of the antiepileptic drug substance vigabatrin in Göttingen mini-pigs is unaffected by co-administration of amino acids.Int J Pharm. 2014 May 15;466(1-2):18-20. doi: 10.1016/j.ijpharm.2014.03.004. Epub 2014 Mar 5. Int J Pharm. 2014. PMID: 24607206
-
Is oral absorption of vigabatrin carrier-mediated?Eur J Pharm Sci. 2015 Mar 10;69:10-8. doi: 10.1016/j.ejps.2014.12.018. Epub 2015 Jan 3. Eur J Pharm Sci. 2015. PMID: 25562534
-
The absorptive flux of the anti-epileptic drug substance vigabatrin is carrier-mediated across Caco-2 cell monolayers.Eur J Pharm Sci. 2014 Jan 23;51:1-10. doi: 10.1016/j.ejps.2013.08.034. Epub 2013 Sep 2. Eur J Pharm Sci. 2014. PMID: 24008184
-
Pharmacokinetic aspects of the anti-epileptic drug substance vigabatrin: focus on transporter interactions.Ther Deliv. 2014 Aug;5(8):927-42. doi: 10.4155/tde.14.55. Ther Deliv. 2014. PMID: 25337649 Review.
-
Vigabatrin in the treatment of infantile spasms in tuberous sclerosis: literature review.J Child Neurol. 1999 Feb;14(2):71-4. doi: 10.1177/088307389901400201. J Child Neurol. 1999. PMID: 10073425 Review.
Cited by
-
Pharmacokinetic Drug-Drug Interactions among Antiepileptic Drugs, Including CBD, Drugs Used to Treat COVID-19 and Nutrients.Int J Mol Sci. 2021 Sep 3;22(17):9582. doi: 10.3390/ijms22179582. Int J Mol Sci. 2021. PMID: 34502487 Free PMC article. Review.
-
Oral and intravenous pharmacokinetics of taurine in sprague-dawley rats: the influence of dose and the possible involvement of the proton-coupled amino acid transporter, PAT1, in oral taurine absorption.Physiol Rep. 2017 Oct;5(19):e13467. doi: 10.14814/phy2.13467. Epub 2017 Oct 16. Physiol Rep. 2017. PMID: 29038364 Free PMC article.
-
Exploring Amino Acid Transporters as Therapeutic Targets for Cancer: An Examination of Inhibitor Structures, Selectivity Issues, and Discovery Approaches.Pharmaceutics. 2024 Jan 30;16(2):197. doi: 10.3390/pharmaceutics16020197. Pharmaceutics. 2024. PMID: 38399253 Free PMC article. Review.
References
-
- Danish Health and Medicines Authority. Produktresumé for Sabrilex, pulver til oral opløsning. Danish Health and Medicines Authority. 2013. Available at http://www.produktresume.dk/docushare/dsweb/View/Collection-115 (accessed December 6, 2013)
-
- Drug information online. Vigabatrin Dosage. U.S. Food and Drug Administration. 2013. Available at http://www.drugs.com/dosage/vigabatrin.html (accessed November 19, 2013)
LinkOut - more resources
Full Text Sources
Other Literature Sources

