The neural crest lineage as a driver of disease heterogeneity in Tuberous Sclerosis Complex and Lymphangioleiomyomatosis
- PMID: 25505789
- PMCID: PMC4243694
- DOI: 10.3389/fcell.2014.00069
The neural crest lineage as a driver of disease heterogeneity in Tuberous Sclerosis Complex and Lymphangioleiomyomatosis
Abstract
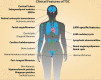
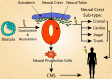
Lymphangioleiomyomatosis (LAM) is a rare neoplastic disease, best characterized by the formation of proliferative nodules that express smooth muscle and melanocytic antigens within the lung parenchyma, leading to progressive destruction of lung tissue and function. The pathological basis of LAM is associated with Tuberous Sclerosis Complex (TSC), a multi-system disorder marked by low-grade tumors in the brain, kidneys, heart, eyes, lung and skin, arising from inherited or spontaneous germ-line mutations in either of the TSC1 or TSC2 genes. LAM can develop either in a patient with TSC (TSC-LAM) or spontaneously (S-LAM), and it is clear that the majority of LAM lesions of both forms are characterized by an inactivating mutation in either TSC1 or TSC2, as in TSC. Despite this genetic commonality, there is considerable heterogeneity in the tumor spectrum of TSC and LAM patients, the basis for which is currently unknown. There is extensive clinical evidence to suggest that the cell of origin for LAM, as well as many of the TSC-associated tumors, is a neural crest cell, a highly migratory cell type with extensive multi-lineage potential. Here we explore the hypothesis that the types of tumors that develop and the tissues that are affected in TSC and LAM are dictated by the developmental timing of TSC gene mutations, which determines the identities of the affected cell types and the size of downstream populations that acquire a mutation. We further discuss the evidence to support a neural crest origin for LAM and TSC tumors, and propose approaches for generating humanized models of TSC and LAM that will allow cell of origin theories to be experimentally tested. Identifying the cell of origin and developing appropriate humanized models is necessary to truly understand LAM and TSC pathology and to establish effective and long-lasting therapeutic approaches for these patients.
Keywords: Lymphangioleiomyomatosis; Tuberous Sclerosis; cell of origin; disease modeling; neural crest.
Figures
Similar articles
-
[18F]Fluorocholine and [18F]Fluoroacetate PET as Imaging Biomarkers to Assess Phosphatidylcholine and Mitochondrial Metabolism in Preclinical Models of TSC and LAM.Clin Cancer Res. 2018 Dec 1;24(23):5925-5938. doi: 10.1158/1078-0432.CCR-17-3693. Epub 2018 Jul 27. Clin Cancer Res. 2018. PMID: 30054282 Free PMC article.
-
Mutation analysis of the TSC1 and TSC2 genes in Japanese patients with pulmonary lymphangioleiomyomatosis.J Hum Genet. 2002;47(1):20-8. doi: 10.1007/s10038-002-8651-8. J Hum Genet. 2002. PMID: 11829138
-
Lymphangioleiomyomatosis Association with Underlying Genotype in Patients with Tuberous Sclerosis Complex.Ann Am Thorac Soc. 2021 May;18(5):815-819. doi: 10.1513/AnnalsATS.202008-911OC. Ann Am Thorac Soc. 2021. PMID: 33171065
-
Pathogenesis of multifocal micronodular pneumocyte hyperplasia and lymphangioleiomyomatosis in tuberous sclerosis and association with tuberous sclerosis genes TSC1 and TSC2.Pathol Int. 2001 Aug;51(8):585-94. doi: 10.1046/j.1440-1827.2001.01242.x. Pathol Int. 2001. PMID: 11564212 Review.
-
A patient with TSC1 germline mutation whose clinical phenotype was limited to lymphangioleiomyomatosis.J Intern Med. 2004 Aug;256(2):166-73. doi: 10.1111/j.1365-2796.2004.01356.x. J Intern Med. 2004. PMID: 15257730 Review.
Cited by
-
Notch transactivates Rheb to maintain the multipotency of TSC-null cells.Nat Commun. 2017 Nov 29;8(1):1848. doi: 10.1038/s41467-017-01845-1. Nat Commun. 2017. PMID: 29184052 Free PMC article.
-
Renal neoplasms in tuberous sclerosis mice are neurocristopathies.iScience. 2021 Jun 4;24(7):102684. doi: 10.1016/j.isci.2021.102684. eCollection 2021 Jul 23. iScience. 2021. PMID: 34222844 Free PMC article.
-
The genomic landscape of tuberous sclerosis complex.Nat Commun. 2017 Jun 15;8:15816. doi: 10.1038/ncomms15816. Nat Commun. 2017. PMID: 28643795 Free PMC article.
-
Advances in physiological and clinical relevance of hiPSC-derived brain models for precision medicine pipelines.Front Cell Neurosci. 2025 Jan 6;18:1478572. doi: 10.3389/fncel.2024.1478572. eCollection 2024. Front Cell Neurosci. 2025. PMID: 39835290 Free PMC article. Review.
-
Lymphangioleiomyomatosis Biomarkers Linked to Lung Metastatic Potential and Cell Stemness.PLoS One. 2015 Jul 13;10(7):e0132546. doi: 10.1371/journal.pone.0132546. eCollection 2015. PLoS One. 2015. PMID: 26167915 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

