Metal-Free Preparation of Cycloalkyl Aryl Sulfides via Di-tert-butyl Peroxide-Promoted Oxidative C(sp3)[BOND]H Bond Thiolation of Cycloalkanes
- PMID: 25505857
- PMCID: PMC4260410
- DOI: 10.1002/adsc.201400032
Metal-Free Preparation of Cycloalkyl Aryl Sulfides via Di-tert-butyl Peroxide-Promoted Oxidative C(sp3)[BOND]H Bond Thiolation of Cycloalkanes
Abstract
A concise thiolation of C(sp3)-H bond of cycloalkanes with diaryl disulfides in the presence of oxidant of di-tert-butylperoxide (DTBP) has been developed. This reaction without using any of metal catalyst, tolerates varieties of disulfides and cycloalkanes substrates, giving good to excellent chemical yields, which provides a useful approach to cycloalkyl aryl sulfides from unactivated cycloalkanes.
Keywords: C(sp3)–H activation; cycloalkane; metal-free; oxidative; thiolation.
Figures



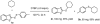



Similar articles
-
Metal-free oxidative C(sp3)-H bond thiolation of ethers with disulfides.Org Lett. 2013 Sep 20;15(18):4654-7. doi: 10.1021/ol402281f. Epub 2013 Aug 30. Org Lett. 2013. PMID: 23987104
-
Metal-free oxidative C(sp3)-H bond functionalization of alkanes and conjugate addition to chromones.Org Lett. 2014 Oct 17;16(20):5342-5. doi: 10.1021/ol502524d. Epub 2014 Sep 25. Org Lett. 2014. PMID: 25255426
-
Metal-free oxidative C(sp(3))-H bond functionalization of alkanes and alkylation-initiated radical 1,2-aryl migration in α,α-diaryl allylic alcohols.Chem Commun (Camb). 2015 Jan 11;51(3):599-602. doi: 10.1039/c4cc07654k. Chem Commun (Camb). 2015. PMID: 25415337
-
Catalytic alkylation of unactivated C(sp3)-H bonds for C(sp3)-C(sp3) bond formation.Chem Soc Rev. 2019 Sep 16;48(18):4921-4942. doi: 10.1039/c9cs00086k. Chem Soc Rev. 2019. PMID: 31403147 Review.
-
Organic Peroxides in Transition-Metal-Free Cyclization and Coupling Reactions (C-C) via Oxidative Transformation.ACS Omega. 2025 Apr 14;10(16):15852-15907. doi: 10.1021/acsomega.4c11574. eCollection 2025 Apr 29. ACS Omega. 2025. PMID: 40321530 Free PMC article. Review.
Cited by
-
Synthesis, structure, and antioxidant activity of methoxy- and hydroxyl-substituted 2'-aminochalcones.Monatsh Chem. 2016;147(10):1747-1757. doi: 10.1007/s00706-016-1812-9. Epub 2016 Aug 26. Monatsh Chem. 2016. PMID: 27729713 Free PMC article.
-
Light-activated hypervalent iodine agents enable diverse aliphatic C-H functionalization.Nat Chem. 2025 Mar;17(3):365-372. doi: 10.1038/s41557-025-01749-4. Epub 2025 Feb 24. Nat Chem. 2025. PMID: 39994489
-
Visible-Light-Driven C-S Bond Formation Based on Electron Donor-Acceptor Excitation and Hydrogen Atom Transfer Combined System.ACS Org Inorg Au. 2021 Jun 22;1(1):23-28. doi: 10.1021/acsorginorgau.1c00007. eCollection 2021 Oct 6. ACS Org Inorg Au. 2021. PMID: 36855634 Free PMC article.
-
C-H Xanthylation: A Synthetic Platform for Alkane Functionalization.J Am Chem Soc. 2016 Oct 26;138(42):13854-13857. doi: 10.1021/jacs.6b09414. Epub 2016 Oct 14. J Am Chem Soc. 2016. PMID: 27739673 Free PMC article.
-
Silver-catalyzed direct thiolation of quinones by activation of aryl disulfides to synthesize quinonyl aryl thioethers.J Org Chem. 2015 May 15;80(10):4919-27. doi: 10.1021/acs.joc.5b00247. Epub 2015 Apr 27. J Org Chem. 2015. PMID: 25894360 Free PMC article.
References
-
- Dua RK, Taylor EW, Phillips RS. J Am Chem Soc. 1993;115:1264–1270.
- Rayner CM. Contemp Org Synth. 1996;3:499–533.
- Hartwig JF. Acc Chem Res. 1998;31:852–860.
- Baird CP, Rayner CM. J Chem Soc Perkin Trans. 1998;1:1973–2003.
- Procter DJ. J Chem Soc Perkin Trans. 1999;1:641–668.
- Ley SV, Thomas AW. Angew Chem Int Ed. 2003;42:5400–5449. - PubMed
- Gangjee A, Zeng Y, Talreja T, McGuire JJ, Kisliuk RL, Queener SF. J Med Chem. 2007;50:3046–3053. - PMC - PubMed
- Beletskaya IP, Ananikov VP. Chem Rev. 2011;111:1596–1636. - PubMed
-
- Li GY, Zheng G, Noonan AF. J Org Chem. 2001;66:8677–8681. - PubMed
- Itoh T, Mase T. Org Lett. 2004;6:4587–4590. - PubMed
- Fernández-Rodríguez MA, Shen Q, Hartwig JF. J Am Chem Soc. 2006;128:2180–2181. - PubMed
- Rout L, Sen TK, Punniyamurthy T. Angew Chem Int Ed. 2007;46:5583–5586. - PubMed
- Ranu BC, Saha A, Jana R. Adv Synth Catal. 2007;349:2690–2696.
- Zhang Y, Ngeow KC, Ying JY. Org Lett. 2007;9:3495–3498. - PubMed
- Rout L, Saha P, Jammi S, Punniyamurthy T. Eur J Org Chem. 2008:640–643.
- Reddy VP, Swapna K, Kumar AV, Rao KR. J Org Chem. 2009;74:3189–3191. - PubMed
- Chen CK, Chen YW, Lin CH, Lin HP, Lee CF. Chem Commun. 2010;46:282–284. - PubMed
- Kovács S, Novák Z. Org Biomol Chem. 2011;9:711–716. - PubMed
- Xu HJ, Liang YF, Zhou XF, Feng YS. Org Biomol Chem. 2012;10:2562–2568. - PubMed
- Bastug G, Nolan SP. J Org Chem. 2013;78:9303–9308. - PubMed
-
- Chu L, Yue X, Qing FL. Org Lett. 2010;12:1644–1647. - PubMed
- Zhang S, Qian P, Zhang M, Hu M, Cheng J. J Org Chem. 2010;75:6732–6735. - PubMed
- Wu Q, Zhao D, Qin X, Lan J, You J. Chem Commun. 2011;47:9188–9190. - PubMed
- Ranjit S, Lee R, Heryadi D, Shen C, Wu JE, Zhang P, Huang KW, Liu X. J Org Chem. 2011;76:8999–9007. - PubMed
- Zhou AX, Liu XY, Yang K, Zhao SC, Liang YM. Org Biomol Chem. 2011;9:5456–5462. - PubMed
- Zou LH, Reball J, Mottweiler J, Bolm C. Chem Commun. 2012;48:11307–11309. - PubMed
- He Z, Luo F, Li Y, Zhu G. Tetrahedron Lett. 2013;54:5907–5910.
- Rosario AR, Casola KK, Oliveira CES, Zeni G. Adv Synth Catal. 2013;355:2960–2966.
-
- Tang RY, Xie YX, Xie YL, Xiang JN, Li JH. Chem Commun. 2011;47:12867–12869. - PubMed
-
-
For selected examples of C(sp3)–H activation, see: Chatani N, Asaumi T, Yorimitsu S, Ikeda T, Kakiuchi F, Murai S. J Am Chem Soc. 2001;123:10935–10941.; DeBoef B, Pastine SJ, Sames D. J Am Chem Soc. 2004;126:6556–6557.; Song CX, Cai GX, Farrell TR, Jiang ZP, Li H, Gan LB, Shi ZJ. Chem Commun. 2009:6002–6004.; Harris JF., Jr J Org Chem. 1981;46:268–271.; Mitroka S, Zimmeck S, Troya D, Tanko JM. J Am Chem Soc. 2010;132:2907–2913.; Antonchick AP, Burgmann L. Angew Chem Int Ed. 2013;52:3267–3271.; Ryu I, Tani A, Fukuyama T, Ravelli D, Fagnoni M, Albini A. Angew Chem Int Ed. 2011;50:1869–1872.; Chuprakov S, Malik JA, Zibinsky M, Fokin VV. J Am Chem Soc. 2011;133:10352–10355.; Ravelli D, Montanaro S, Zema M, Fagnoni M, Albini A. Adv Synth Catal. 2011;353:3295–3300.; Samanta R, Matcha K, Antonchick AP. Eur J Org Chem. 2013:5769–5804..
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
