Molecular magnetic resonance imaging of brain-immune interactions
- PMID: 25505871
- PMCID: PMC4245913
- DOI: 10.3389/fncel.2014.00389
Molecular magnetic resonance imaging of brain-immune interactions
Abstract
Although the blood-brain barrier (BBB) was thought to protect the brain from the effects of the immune system, immune cells can nevertheless migrate from the blood to the brain, either as a cause or as a consequence of central nervous system (CNS) diseases, thus contributing to their evolution and outcome. Accordingly, as the interface between the CNS and the peripheral immune system, the BBB is critical during neuroinflammatory processes. In particular, endothelial cells are involved in the brain response to systemic or local inflammatory stimuli by regulating the cellular movement between the circulation and the brain parenchyma. While neuropathological conditions differ in etiology and in the way in which the inflammatory response is mounted and resolved, cellular mechanisms of neuroinflammation are probably similar. Accordingly, neuroinflammation is a hallmark and a decisive player of many CNS diseases. Thus, molecular magnetic resonance imaging (MRI) of inflammatory processes is a central theme of research in several neurological disorders focusing on a set of molecules expressed by endothelial cells, such as adhesion molecules (VCAM-1, ICAM-1, P-selectin, E-selectin, …), which emerge as therapeutic targets and biomarkers for neurological diseases. In this review, we will present the most recent advances in the field of preclinical molecular MRI. Moreover, we will discuss the possible translation of molecular MRI to the clinical setting with a particular emphasis on myeloperoxidase imaging, autologous cell tracking, and targeted iron oxide particles (USPIO, MPIO).
Keywords: Alzheimer; antibody; hemorrhage; inflammation; lymphocytes; microparticles; multiple sclerosis; stroke.
Figures

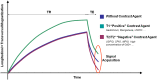


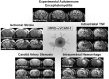

Similar articles
-
Molecular Magnetic Resonance Imaging of Endothelial Activation in the Central Nervous System.Theranostics. 2018 Feb 2;8(5):1195-1212. doi: 10.7150/thno.22662. eCollection 2018. Theranostics. 2018. PMID: 29507614 Free PMC article. Review.
-
Structural pathways for macromolecular and cellular transport across the blood-brain barrier during inflammatory conditions. Review.Histol Histopathol. 2004 Apr;19(2):535-64. doi: 10.14670/HH-19.535. Histol Histopathol. 2004. PMID: 15024715 Review.
-
Imaging of inflammation in the peripheral and central nervous system by magnetic resonance imaging.Neuroscience. 2009 Feb 6;158(3):1151-60. doi: 10.1016/j.neuroscience.2008.06.045. Epub 2008 Jun 26. Neuroscience. 2009. PMID: 18651996 Review.
-
Immuno-MRI for Stroke Diagnosis and Prognosis.Neuroscience. 2024 Jul 9;550:53-61. doi: 10.1016/j.neuroscience.2023.12.007. Epub 2023 Dec 21. Neuroscience. 2024. PMID: 38141809 Review.
-
Immune cell trafficking across the barriers of the central nervous system in multiple sclerosis and stroke.Biochim Biophys Acta. 2016 Mar;1862(3):461-71. doi: 10.1016/j.bbadis.2015.10.018. Epub 2015 Oct 23. Biochim Biophys Acta. 2016. PMID: 26527183 Review.
Cited by
-
The Brain-Heart Axis: Neuroinflammatory Interactions in Cardiovascular Disease.Curr Cardiol Rep. 2023 Dec;25(12):1745-1758. doi: 10.1007/s11886-023-01990-8. Epub 2023 Nov 23. Curr Cardiol Rep. 2023. PMID: 37994952 Free PMC article. Review.
-
Improved Reperfusion and Vasculoprotection by the Poly(ADP-Ribose)Polymerase Inhibitor PJ34 After Stroke and Thrombolysis in Mice.Mol Neurobiol. 2018 Dec;55(12):9156-9168. doi: 10.1007/s12035-018-1063-3. Epub 2018 Apr 12. Mol Neurobiol. 2018. PMID: 29651748
-
Multimodal neuroimaging computing: a review of the applications in neuropsychiatric disorders.Brain Inform. 2015 Sep;2(3):167-180. doi: 10.1007/s40708-015-0019-x. Epub 2015 Aug 29. Brain Inform. 2015. PMID: 27747507 Free PMC article. Review.
-
In Vivo Molecular MRI of ICAM-1 Expression on Endothelium and Leukocytes from Subacute to Chronic Stages After Experimental Stroke.Transl Stroke Res. 2017 May 16;8(5):440-8. doi: 10.1007/s12975-017-0536-4. Online ahead of print. Transl Stroke Res. 2017. PMID: 28509283 Free PMC article.
-
Longitudinal Molecular Magnetic Resonance Imaging of Endothelial Activation after Severe Traumatic Brain Injury.J Clin Med. 2019 Jul 30;8(8):1134. doi: 10.3390/jcm8081134. J Clin Med. 2019. PMID: 31366109 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

