Pharmacokinetic non-interaction analysis in a fixed-dose formulation in combination of atorvastatin and ezetimibe
- PMID: 25505887
- PMCID: PMC4245888
- DOI: 10.3389/fphar.2014.00261
Pharmacokinetic non-interaction analysis in a fixed-dose formulation in combination of atorvastatin and ezetimibe
Abstract
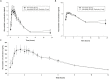
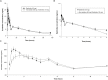
Recent clinical research has shown that atorvastatin (ATO) in combination with cholesterol absorption inhibitor ezetimibe (EZE) significantly reduces LDL-C level in patients with hypercholesterolemia, showing a superior lipid-lowering efficacy compared to statin alone. With no information currently available on the interaction between the two drugs, a pharmacokinetic study was conducted to investigate the influence of EZE on ATO and conversely when the two drugs were coadministered. The purpose of this study was to investigate the presence of differences in the pharmacokinetic profiles of capsules containing ATO 80 mg, EZE 10 mg or the combination of both 80/10 mg administered to healthy Mexican volunteers. This was a randomized, three-period, six-sequences crossover study. 36 eligible subjects aged between 20 to 50 years were included. Blood samples were collected up to 96 h after dosing, and pharmacokinetic parameters were obtained by non-compartmental analysis. Adverse events were evaluated based on subject interviews and physical examinations. Area under the concentration-time curve (AUC) and maximum plasma drug concentration (Cmax) were measured for each drug alone or together and tested for bioequivalence-based hypothesis. The estimation computed (90% confidence intervals) for AUC and Cmax, were 96.04% (85.88-107.42%) and 97.04% (82.36-114.35%), respectively for ATO-EZE combination versus ATO alone, while 84.42% (77.19-92.32%) and 95.60% (82.43-110.88%), respectively, for ATO-EZE combination versus EZE alone were estimated. These results suggest that ATO and EZE have no relevant pharmacokinetic drug-drug interaction.
Keywords: LC-MS-MS; atorvastatin; ezetimibe; pharmacokinetic drug–drug interaction; statins.
Figures
Similar articles
-
Pharmacokinetic drug interaction between atorvastatin and ezetimibe in healthy Korean volunteers.Transl Clin Pharmacol. 2017 Dec;25(4):202-208. doi: 10.12793/tcp.2017.25.4.202. Epub 2017 Dec 20. Transl Clin Pharmacol. 2017. PMID: 32095476 Free PMC article.
-
Absence of a significant pharmacokinetic interaction between atorvastatin and fenofibrate: a randomized, crossover, study of a fixed-dose formulation in healthy Mexican subjects.Front Pharmacol. 2015 Jan 29;6:4. doi: 10.3389/fphar.2015.00004. eCollection 2015. Front Pharmacol. 2015. PMID: 25688207 Free PMC article.
-
Bioequivalence of an ezetimibe/simvastatin combination tablet and coadministration of ezetimibe and simvastatin as separate tablets in healthy subjects.Int J Clin Pharmacol Ther. 2006 Feb;44(2):83-92. doi: 10.5414/cpp44083. Int J Clin Pharmacol Ther. 2006. PMID: 16502768 Clinical Trial.
-
Ezetimibe: a first-in-class, novel cholesterol absorption inhibitor.Cardiovasc Drug Rev. 2003 Winter;21(4):293-312. doi: 10.1111/j.1527-3466.2003.tb00123.x. Cardiovasc Drug Rev. 2003. PMID: 14647533 Review.
-
Understanding practice patterns and low-density lipoprotein cholesterol goal attainment implications of switching patients from simvastatin in a health plan setting.Am J Manag Care. 2007 Dec;13 Suppl 10:S276-81. Am J Manag Care. 2007. PMID: 18095778 Review.
Cited by
-
Pharmacokinetic drug interaction between atorvastatin and ezetimibe in healthy Korean volunteers.Transl Clin Pharmacol. 2017 Dec;25(4):202-208. doi: 10.12793/tcp.2017.25.4.202. Epub 2017 Dec 20. Transl Clin Pharmacol. 2017. PMID: 32095476 Free PMC article.
-
Enhancing Atorvastatin In Vivo Oral Bioavailability in the Presence of Inflammatory Bowel Disease and Irritable Bowel Syndrome Using Supercritical Fluid Technology Guided by wbPBPK Modeling in Rat and Human.AAPS PharmSciTech. 2022 May 18;23(5):148. doi: 10.1208/s12249-022-02302-z. AAPS PharmSciTech. 2022. PMID: 35585214
-
Fixed-dose combination orally disintegrating tablets to treat cardiovascular disease: formulation, in vitro characterization and physiologically based pharmacokinetic modeling to assess bioavailability.Drug Des Devel Ther. 2017 Mar 16;11:811-826. doi: 10.2147/DDDT.S126035. eCollection 2017. Drug Des Devel Ther. 2017. PMID: 28352156 Free PMC article. Clinical Trial.
-
Comparing the combination therapy of ezetimibe and atorvastatin with atorvastatin monotherapy for regulating blood lipids: a systematic review and meta-analyse.Lipids Health Dis. 2018 Oct 17;17(1):239. doi: 10.1186/s12944-018-0880-8. Lipids Health Dis. 2018. PMID: 30326894 Free PMC article.
-
Absence of a significant pharmacokinetic interaction between atorvastatin and fenofibrate: a randomized, crossover, study of a fixed-dose formulation in healthy Mexican subjects.Front Pharmacol. 2015 Jan 29;6:4. doi: 10.3389/fphar.2015.00004. eCollection 2015. Front Pharmacol. 2015. PMID: 25688207 Free PMC article.
References
-
- Bahrami G., Mohammadi B., Khatabi P. M., Farzaei M. H., Majnooni M. B., Bahoosh S. R. (2010). Application of one-step liquid chromatography-electrospray tandem MS/MS and collision-induced dissociation to quantification of ezetimibe and identification of its glucuronated metabolite in human serum: a pharmacokinetic study. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 15 2789–2795 10.1016/j.jchromb.2010.08.023 - DOI - PubMed
-
- Ballantyne C. M., Abate N., Yuan Z., King T. R., Palmisano J. (2005). Dose-comparison study of the combination of ezetimibe and simvastatin (Vytorin) versus atorvastatin in patients with hypercholesterolemia: the Vytorin Versus Atorvastatin (VYVA) study. Am. Heart J. 149 464–473 10.1016/j.ahj.2004.11.023 - DOI - PubMed
-
- Bhatt K. K., Shankar M. B., Patel J. B., Christian M. C. (2010). Simultaneous estimation of atorvastatin calcium and ezetimibe in tablet by RP-HPLC method. Int. J. Pharm. Appl. Sci. 1 114–117 10.4172/2153-2435.1000111 - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

