Pien Tze Huang Overcomes Multidrug Resistance and Epithelial-Mesenchymal Transition in Human Colorectal Carcinoma Cells via Suppression of TGF-β Pathway
- PMID: 25505925
- PMCID: PMC4253702
- DOI: 10.1155/2014/679436
Pien Tze Huang Overcomes Multidrug Resistance and Epithelial-Mesenchymal Transition in Human Colorectal Carcinoma Cells via Suppression of TGF-β Pathway
Abstract
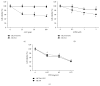
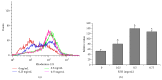
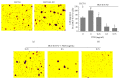
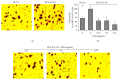
The traditional Chinese medicine formula Pien Tze Huang (PZH) has long been used as a folk remedy for cancer. To elucidate the mode of action of PZH against cancer, in the present study we used a 5-FU resistant human colorectal carcinoma cell line (HCT-8/5-FU) to evaluate the effects of PZH on multidrug resistance (MDR) and epithelial-mesenchymal transition (EMT) as well as the activation of TGF-β pathway. We found that PZH dose-dependently inhibited the viability of HCT-8/5-FU cells which were insensitive to treatment of 5-FU and ADM, demonstrating the ability of PZH to overcome chemoresistance. Furthermore, PZH increased the intercellular accumulation of Rhodamine-123 and downregulated the expression of ABCG2 in HCT-8/5-FU cells. In addition, drug resistance induced the process of EMT in HCT-8 cells as evidenced by EMT-related morphological changes and alteration in the expression of EMT-regulatory factors, which however was neutralized by PZH treatment. Moreover, PZH inhibited MDR/EMT-enhanced migration and invasion capabilities of HCT-8 cells in a dose-dependent manner and suppressed MDR-induced activation of TGF-β signaling in HCT-8/5-FU cells. Taken together, our study suggests that PZH can effectively overcome MDR and inhibit EMT in human colorectal carcinoma cells via suppression of the TGF-β pathway.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources

