Epstein-Barr virus and multiple sclerosis: potential opportunities for immunotherapy
- PMID: 25505955
- PMCID: PMC4237030
- DOI: 10.1038/cti.2014.25
Epstein-Barr virus and multiple sclerosis: potential opportunities for immunotherapy
Abstract
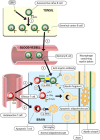
Multiple sclerosis (MS) is a common chronic inflammatory demyelinating disease of the central nervous system (CNS) causing progressive disability. Many observations implicate Epstein-Barr virus (EBV) in the pathogenesis of MS, namely universal EBV seropositivity, high anti-EBV antibody levels, alterations in EBV-specific CD8(+) T-cell immunity, increased spontaneous EBV-induced transformation of peripheral blood B cells, increased shedding of EBV from saliva and accumulation of EBV-infected B cells and plasma cells in the brain. Several mechanisms have been postulated to explain the role of EBV in the development of MS including cross-reactivity between EBV and CNS antigens, bystander damage to the CNS by EBV-specific CD8(+) T cells, activation of innate immunity by EBV-encoded small RNA molecules in the CNS, expression of αB-crystallin in EBV-infected B cells leading to a CD4(+) T-cell response against oligodendrocyte-derived αB-crystallin and EBV infection of autoreactive B cells, which produce pathogenic autoantibodies and provide costimulatory survival signals to autoreactive T cells in the CNS. The rapidly accumulating evidence for a pathogenic role of EBV in MS provides ground for optimism that it might be possible to prevent and cure MS by effectively controlling EBV infection through vaccination, antiviral drugs or treatment with EBV-specific cytotoxic CD8(+) T cells. Adoptive immunotherapy with in vitro-expanded autologous EBV-specific CD8(+) T cells directed against viral latent proteins was recently used to treat a patient with secondary progressive MS. Following the therapy, there was clinical improvement, decreased disease activity on magnetic resonance imaging and reduced intrathecal immunoglobulin production.
Figures
Similar articles
-
Epstein-Barr Virus-Specific CD8 T Cells Selectively Infiltrate the Brain in Multiple Sclerosis and Interact Locally with Virus-Infected Cells: Clue for a Virus-Driven Immunopathological Mechanism.J Virol. 2019 Nov 26;93(24):e00980-19. doi: 10.1128/JVI.00980-19. Print 2019 Dec 15. J Virol. 2019. PMID: 31578295 Free PMC article.
-
Epstein-Barr virus-specific adoptive immunotherapy for progressive multiple sclerosis.Mult Scler. 2014 Oct;20(11):1541-4. doi: 10.1177/1352458514521888. Epub 2014 Feb 3. Mult Scler. 2014. PMID: 24493474 Free PMC article.
-
Epstein-Barr virus-specific T cell therapy for progressive multiple sclerosis.JCI Insight. 2018 Nov 15;3(22):e124714. doi: 10.1172/jci.insight.124714. JCI Insight. 2018. PMID: 30429369 Free PMC article. Clinical Trial.
-
Preventing and curing multiple sclerosis by controlling Epstein-Barr virus infection.Autoimmun Rev. 2009 Jun;8(7):563-8. doi: 10.1016/j.autrev.2009.01.017. Epub 2009 Jan 30. Autoimmun Rev. 2009. PMID: 19254880 Review.
-
The essential role of Epstein-Barr virus in the pathogenesis of multiple sclerosis.Neuroscientist. 2011 Aug;17(4):351-67. doi: 10.1177/1073858410381531. Epub 2010 Nov 12. Neuroscientist. 2011. PMID: 21075971 Free PMC article. Review.
Cited by
-
Sustained Clinical Improvement in a Subset of Patients With Progressive Multiple Sclerosis Treated With Epstein-Barr Virus-Specific T Cell Therapy.Front Neurol. 2021 Mar 15;12:652811. doi: 10.3389/fneur.2021.652811. eCollection 2021. Front Neurol. 2021. PMID: 33790852 Free PMC article.
-
An evaluation of the recognised systemic inflammatory biomarkers of chronic sub-optimal inflammation provides evidence for inflammageing (IFA) during multiple sclerosis (MS).Immun Ageing. 2021 Apr 14;18(1):18. doi: 10.1186/s12979-021-00225-0. Immun Ageing. 2021. PMID: 33853634 Free PMC article. Review.
-
Multiple sclerosis and drug discovery: A work of translation.EBioMedicine. 2021 Jun;68:103392. doi: 10.1016/j.ebiom.2021.103392. Epub 2021 May 24. EBioMedicine. 2021. PMID: 34044219 Free PMC article. Review.
-
MicroRNAs in blood and cerebrospinal fluid as diagnostic biomarkers of multiple sclerosis and to monitor disease progression.Neural Regen Res. 2020 Apr;15(4):606-619. doi: 10.4103/1673-5374.266905. Neural Regen Res. 2020. PMID: 31638082 Free PMC article. Review.
-
Epstein-Barr Virus and the Origin of Myalgic Encephalomyelitis or Chronic Fatigue Syndrome.Front Immunol. 2021 Nov 15;12:656797. doi: 10.3389/fimmu.2021.656797. eCollection 2021. Front Immunol. 2021. PMID: 34867935 Free PMC article. Review.
References
-
- Pender MP. Neurology 4: multiple sclerosis. Med J Aust. 2000;172:556–562. - PubMed
-
- Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. - PubMed
-
- Sospedra M, Martin R. Immunology of multiple sclerosis. Annu Rev Immunol. 2005;23:683–747. - PubMed
-
- Pender MP, Greer JM. Immunology of multiple sclerosis. Curr Allergy Asthma Rep. 2007;7:285–292. - PubMed
-
- Ascherio A, Munger KL. Epstein–Barr virus infection and multiple sclerosis: a review. J Neuroimmune Pharmacol. 2010;5:271–277. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
