Increase in DNA vaccine efficacy by virosome delivery and co-expression of a cytolytic protein
- PMID: 25505966
- PMCID: PMC4232068
- DOI: 10.1038/cti.2014.13
Increase in DNA vaccine efficacy by virosome delivery and co-expression of a cytolytic protein
Abstract
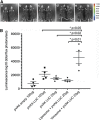
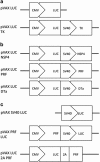
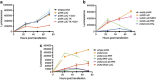
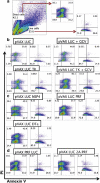
The potential of DNA vaccines has not been realised due to suboptimal delivery, poor antigen expression and the lack of localised inflammation, essential for antigen presentation and an effective immune response to the immunogen. Initially, we examined the delivery of a DNA vaccine encoding a model antigen, luciferase (LUC), to the respiratory tract of mice by encapsulation in a virosome. Virosomes that incorporated influenza virus haemagglutinin effectively delivered DNA to cells in the mouse respiratory tract and resulted in antigen expression and systemic and mucosal immune responses to the immunogen after an intranasal (IN) prime/intradermal (ID) boost regimen, whereas a multidose ID regimen only generated systemic immunity. We also examined systemic immune responses to LUC after ID vaccination with a DNA vaccine, which also encoded one of the several cytolytic or toxic proteins. Although the herpes simplex virus thymidine kinase, in the presence of the prodrug, ganciclovir, resulted in cell death, this failed to increase the humoral or cell-mediated immune responses. In contrast, the co-expression of LUC with the rotavirus non-structural protein 4 (NSP4) protein or a mutant form of mouse perforin, proteins which are directly cytolytic, resulted in increased LUC-specific humoral and cell-mediated immunity. On the other hand, co-expression of LUC with diphtheria toxin subunit A or overexpression of perforin or NSP4 resulted in a lower level of immunity. In summary, the efficacy of DNA vaccines can be improved by targeted IN delivery of DNA or by the induction of cell death in vaccine-targeted cells after ID delivery.
Figures



Similar articles
-
Induction of antigen-positive cell death by the expression of perforin, but not DTa, from a DNA vaccine enhances the immune response.Immunol Cell Biol. 2014 Apr;92(4):359-67. doi: 10.1038/icb.2013.93. Epub 2013 Dec 10. Immunol Cell Biol. 2014. PMID: 24323081
-
Intradermal delivery of DNA encoding HCV NS3 and perforin elicits robust cell-mediated immunity in mice and pigs.Gene Ther. 2016 Jan;23(1):26-37. doi: 10.1038/gt.2015.86. Epub 2015 Oct 1. Gene Ther. 2016. PMID: 26262584
-
Head-to-head comparison of four nonadjuvanted inactivated cell culture-derived influenza vaccines: effect of composition, spatial organization and immunization route on the immunogenicity in a murine challenge model.Vaccine. 2008 Dec 2;26(51):6555-63. doi: 10.1016/j.vaccine.2008.09.057. Vaccine. 2008. PMID: 18848856
-
The virosome concept for influenza vaccines.Vaccine. 2005 Jul 8;23 Suppl 1:S26-38. doi: 10.1016/j.vaccine.2005.04.026. Vaccine. 2005. PMID: 16026906 Review.
-
Virosomes for antigen and DNA delivery.Adv Drug Deliv Rev. 2005 Jan 10;57(3):451-63. doi: 10.1016/j.addr.2004.09.005. Adv Drug Deliv Rev. 2005. PMID: 15560951 Review.
Cited by
-
Single-Dose Vaccination with a Hepatotropic Adeno-associated Virus Efficiently Localizes T Cell Immunity in the Liver with the Potential To Confer Rapid Protection against Hepatitis C Virus.J Virol. 2019 Sep 12;93(19):e00202-19. doi: 10.1128/JVI.00202-19. Print 2019 Oct 1. J Virol. 2019. PMID: 31292249 Free PMC article.
-
Pre-clinical antigenicity studies of an innovative multivalent vaccine for human visceral leishmaniasis.PLoS Negl Trop Dis. 2017 Nov 27;11(11):e0005951. doi: 10.1371/journal.pntd.0005951. eCollection 2017 Nov. PLoS Negl Trop Dis. 2017. PMID: 29176865 Free PMC article.
-
Cytolytic DNA vaccine encoding lytic perforin augments the maturation of- and antigen presentation by- dendritic cells in a time-dependent manner.Sci Rep. 2017 Aug 17;7(1):8530. doi: 10.1038/s41598-017-08063-1. Sci Rep. 2017. PMID: 28819257 Free PMC article.
-
Bioinspired and Biomimetic Nanotherapies for the Treatment of Infectious Diseases.Front Pharmacol. 2019 Jul 5;10:751. doi: 10.3389/fphar.2019.00751. eCollection 2019. Front Pharmacol. 2019. PMID: 31333467 Free PMC article. Review.
-
Mucosal Vaccine Approaches for Prevention of HIV and SIV Transmission.Curr Immunol Rev. 2019;15(1):102-122. doi: 10.2174/1573395514666180605092054. Curr Immunol Rev. 2019. PMID: 31452652 Free PMC article.
References
-
- Hobson P, Barnfield C, Barnes A, Klavinskis LS. Mucosal immunization with DNA vaccines. Methods. 2003;31:217–224. - PubMed
-
- Ranasinghe C, Ramshaw IA. Genetic heterologous prime-boost vaccination strategies for improved systemic and mucosal immunity. Exp Rev Vacc. 2009;8:1171–1181. - PubMed
-
- Mestecky J. The common mucosal immune system and current strategies for induction of immune responses in external secretions. J Clin Immunol. 1987;7:265–276. - PubMed
-
- Cusi MG. Applications of influenza virosomes as a delivery system. Hum Vaccin. 2006;2:1–7. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
