Enantioselective Synthesis of the Tricyclic Core of FR901483 Featuring a Rh-Catalyzed [2+2+2] Cycloaddition
- PMID: 25506094
- PMCID: PMC4263289
- DOI: 10.1055/s-0032-1316786
Enantioselective Synthesis of the Tricyclic Core of FR901483 Featuring a Rh-Catalyzed [2+2+2] Cycloaddition
Abstract
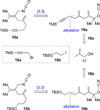
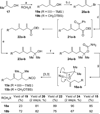
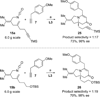
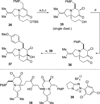
An efficient approach to the tricyclic framework of FR901483 is described. The sequence features a [3, 3]-sigmatropic rearrangement of a cyanate into an isocyanate, followed by its subsequent asymmetric rhodium-catalyzed [2+2+2] cycloaddition with a terminal alkyne for the synthesis of the indolizidine core. The aza-tricyclic core is completed using an intramolecular benzoin reaction to close the last ring of the natural product. Through a model study of the key cycloaddition, we evaluated the impact of different substituents on the tether of the alkenyl isocyanate.
Figures


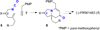


References
-
-
Daly JW. J. Med. Chem. 2003;46:445. Daly JW, Spande TF, Garraffo HM. J. Nat. Prod. 2005;68:1556. For reviews of recent syntheses, see: Michael JP. Nat. Prod. Rep. 2000;17:579. Michael JP. Nat. Prod. Rep. 2002;20:458. Michael JP. Nat. Prod. Rep. 2005;22:603. Michael JP. Nat. Prod. Rep. 2007;24:191. Michael JP. Nat. Prod. Rep. 2008;25:139.
-
-
- Sakamoto K, Tsujii E, Abe F, Nakanishi T, Yamashita M, Shigematsu N, Izumi S, Okuhara M. J. Antibiot. 1996;49:37. - PubMed
-
-
For a review on the biology and the synthesis of FR901483, see: Bonjoch J, Diaba F. In: Studies in Natural Products Chemistry, Bioactive Natural Products (Part L), Volume 32. Atta-ur-Rahman Ed, Elsevier BV., editors. The Netherlands: Amsterdam; 2005. pp. 3–60.
-
-
-
Snider BB, Lin H. J. Am. Chem. Soc. 1999;121:7778. Scheffer G, Seike H, Sorensen EJ. Angew. Chem. Int. Ed. 2000;39:4593. Ousmer M, Braun NA, Ciufolini MA. Org. Lett. 2001;3:765. Maeng J-H, Funk RL. Org. Lett. 2001;3:1125. Kan T, Fujimoto T, Ieda S, Asoh Y, Kitaoka H, Fukuyama T. Org. Lett. 2004;6:2729. Brummond KM, Hong S-P. J. Org. Chem. 2005;70:907. Carson CA, Kerr MA. Org. Lett. 2009;11:777. See also: Ousmer M, Braun NA, Bavoux C, Perrin M, Ciufolini MA. J. Am. Chem. Soc. 2001;123:7534. Ieda S, Asoh Y, Fujimoto T, Kitaoka H, Kan T, Fukuyama T. Heterocycles. 2009;79:721. Ieda S, Kan T, Fukuyama T. Tetrahedron Lett. 2010;51:4027.
-
-
-
For synthetic approaches leading to the tricyclic framework of FR901483 see: Yamazaki N, Suzuki H, Kibayashi C. J. Org. Chem. 1997;62:8280. Wardrop DJ, Zhang W. Org. Lett. 2001;3:2353. Suzuki H, Yamazaki N, Kibayashi C. Tetrahedron Lett. 2001;42:3013. Bonjoch J, Diaba F, Puigbó G, Peidró E, Solé D. Tetrahedron Lett. 2003;44:8387. Panchaud P, Ollivier C, Renaud P, Zigmantas S. J. Org. Chem. 2004;69:2755. Kropf JE, Meigh IC, Bebbington WP, Weinreb SM. J. Org. Chem. 2006;71:2046. Simila STM, Reichelt A, Martin SF. Tetrahedron Lett. 2006;47:2933. Kaden S, Reissig H-U. Org. Lett. 2006;8:4763. Asari A, Angelov P, Auty JM, Hayes C. Tetrahedron Lett. 2007;48:2631. Seike H, Sorensen E. J. Synlett. 2008:695.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
