Splicing modulation therapy in the treatment of genetic diseases
- PMID: 25506237
- PMCID: PMC4259397
- DOI: 10.2147/TACG.S71506
Splicing modulation therapy in the treatment of genetic diseases
Abstract
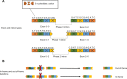
Antisense-mediated splicing modulation is a tool that can be exploited in several ways to provide a potential therapy for rare genetic diseases. This approach is currently being tested in clinical trials for Duchenne muscular dystrophy and spinal muscular atrophy. The present review outlines the versatility of the approach to correct cryptic splicing, modulate alternative splicing, restore the open reading frame, and induce protein knockdown, providing examples of each. Finally, we outline a possible path forward toward the clinical application of this approach for a wide variety of inherited rare diseases.
Keywords: alternative splicing; antisense oligonucleotides; cryptic splicing; splicing; therapy.
Figures

References
-
- Kaplan JC, Hamroun D. The 2014 version of the gene table of monogenic neuromuscular disorders (nuclear genome) Neuromuscul Disord. 2013;23(12):1081–1111. - PubMed
-
- Bertrand AT, Chikhaoui K, Yaou RB, Bonne G. Clinical and genetic heterogeneity in laminopathies. Biochem Soc Trans. 2011;39(6):1687–1692. - PubMed
-
- Goemans NM, Tulinius M, van den Akker JT, et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N Engl J Med. 2011;364(16):1513–1522. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

