Does the presence of coexisting diseases modulate the effectiveness of a low-dose estrogen/progestin, ethinylestradiol/drospirenone combination tablet in dysmenorrhea? Reanalysis of two randomized studies in Japanese women
- PMID: 25506249
- PMCID: PMC4259553
- DOI: 10.2147/IJWH.S70935
Does the presence of coexisting diseases modulate the effectiveness of a low-dose estrogen/progestin, ethinylestradiol/drospirenone combination tablet in dysmenorrhea? Reanalysis of two randomized studies in Japanese women
Abstract
Background: The purpose of this study was to investigate the effectiveness of a combination of ethinylestradiol (EE) and 0.02 mg/drospirenone (DRSP) 3 mg in Japanese women with dysmenorrhea and in particular to determine whether or not the presence of specific coexisting organic diseases (eg, endometriosis, uterine fibroids, uterine adenomyosis) has an impact on treatment.
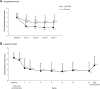
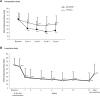
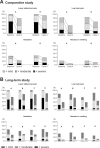
Methods and results: Four hundred and ten patients with dysmenorrhea aged 20 years or older (315 without coexisting organic disease, 28 with endometriosis, 37 with uterine fibroids, and 46 with uterine adenomyosis [some patients had multiple coexisting organic diseases]) were enrolled and treated with EE/DRSP in either a 16-week comparator study or a 52-week long-term safety study. Evaluations included changes in total dysmenorrhea score, visual analog scale for dysmenorrhea, severity of symptoms, hormone levels, endometrial thickness, and safety outcomes. In both studies, the total dysmenorrhea score was significantly (P<0.001) decreased from baseline during treatment with EE/DRSP. Time-dependent changes in visual analog score for dysmenorrhea and alleviation of symptoms, such as lower abdominal pain, low back pain (lumbago), headache, and nausea/vomiting, were similar in all patient groups with and without any specific coexisting organic diseases. These improvements with EE/DRSP were observed for both short-term (16 weeks) and long-term (52 weeks) use. These effects were associated with suppressed increases in serum estradiol and progesterone levels and decreased endometrial thickness. The safety profile of EE/DRSP was similar in all patients, irrespective of the presence of coexisting organic diseases.
Conclusion: EE/DRSP may be prescribed for patients with dysmenorrhea irrespective of the presence of any specific coexisting organic diseases.
Keywords: drospirenone; dysmenorrhea; ethinylestradiol; oral contraceptive; organic disease.
Figures


References
-
- French L. Dysmenorrhea. Am Fam Physician. 2005;71:285–291. - PubMed
-
- Dawood MY. Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol. 2006;108:428–441. - PubMed
-
- Harel Z. Dysmenorrhea in adolescents and young adults: from pathophysiology to pharmacological treatments and management strategies. Expert Opin Pharmacother. 2008;9:2661–2672. - PubMed
-
- Rapkin AJ, Winer S. Drospirenone: a novel progestin. Expert Opin Pharmacother. 2007;8:989–999. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

