The heterodimeric structure of heterogeneous nuclear ribonucleoprotein C1/C2 dictates 1,25-dihydroxyvitamin D-directed transcriptional events in osteoblasts
- PMID: 25506471
- PMCID: PMC4261231
- DOI: 10.1038/boneres.2014.11
The heterodimeric structure of heterogeneous nuclear ribonucleoprotein C1/C2 dictates 1,25-dihydroxyvitamin D-directed transcriptional events in osteoblasts
Abstract
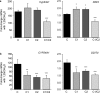
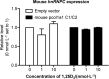
Heterogeneous nuclear ribonucleoprotein (hnRNP) C plays a key role in RNA processing. More recently hnRNP C has also been shown to function as a DNA binding protein exerting a dominant-negative effect on transcriptional responses to the vitamin D hormone,1,25-dihydroxyvitamin D (1,25(OH)2D), via interaction in cis with vitamin D response elements (VDREs). The physiologically active form of human hnRNPC is a tetramer of hnRNPC1 (huC1) and C2 (huC2) subunits known to be critical for specific RNA binding activity in vivo, yet the requirement for heterodimerization of huC1 and C2 in DNA binding and downstream action is not well understood. While over-expression of either huC1 or huC2 alone in mouse osteoblastic cells did not suppress 1,25(OH)2D-induced transcription, over-expression of huC1 and huC2 in combination using a bone-specific polycistronic vector successfully suppressed 1,25(OH)2D-mediated induction of osteoblast target gene expression. Over-expression of either huC1 or huC2 in human osteoblasts was sufficient to confer suppression of 1,25(OH)2D-mediated transcription, indicating the ability of transfected huC1 and huC2 to successfully engage as heterodimerization partners with endogenously expressed huC1 and huC2. The failure of the chimeric combination of mouse and human hnRNPCs to impair 1,25(OH)2D-driven gene expression in mouse cells was structurally predicted, owing to the absence of the last helix in the leucine zipper (LZ) heterodimerization domain of hnRNPC gene product in lower species, including the mouse. These results confirm that species-specific heterodimerization of hnRNPC1 and hnRNPC2 is a necessary prerequisite for DNA binding and down-regulation of 1,25(OH)2D-VDR-VDRE-directed gene transactivation in osteoblasts.
Figures





References
-
- Haussler MR, Whitfield GK, Kaneko I. Molecular mechanisms of vitamin d action. Calcif Tissue Int. 2013;92:77–98. - PubMed
-
- Chen H, Hu B, Allegretto EA, Adams JS. The vitamin D response element-binding protein. A novel dominant-negative regulator of vitamin D-directed transactivation. J Biol Chem. 2000;275:35557–35564. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

