Combinations of immunotherapy and radiation in cancer therapy
- PMID: 25506582
- PMCID: PMC4246656
- DOI: 10.3389/fonc.2014.00325
Combinations of immunotherapy and radiation in cancer therapy
Abstract
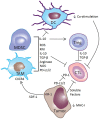
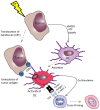
The immune system has the ability to recognize and specifically reject tumors, and tumors only become clinically apparent once they have evaded immune destruction by creating an immunosuppressive tumor microenvironment. Radiotherapy (RT) can cause immunogenic tumor cell death resulting in cross-priming of tumor-specific T-cells, acting as an in situ tumor vaccine; however, RT alone rarely induces effective anti-tumor immunity resulting in systemic tumor rejection. Immunotherapy can complement RT to help overcome tumor-induced immune suppression, as demonstrated in pre-clinical tumor models. Here, we provide the rationale for combinations of different immunotherapies and RT, and review the pre-clinical and emerging clinical evidence for these combinations in the treatment of cancer.
Keywords: abscopal effect; clinical trials; immunotherapy; ionizing radiation; microenvironment; radiotherapy; tumor immunity.
Figures
References
-
- Burnet M. Immunological factors in the process of carcinogenesis. Br Med Bull (1964) 20:154–8. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

