Multivalency in the inhibition of oxidative protein folding by arsenic(III) species
- PMID: 25506675
- PMCID: PMC4303313
- DOI: 10.1021/bi501360e
Multivalency in the inhibition of oxidative protein folding by arsenic(III) species
Abstract
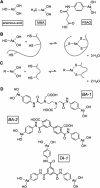
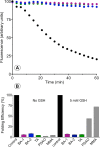
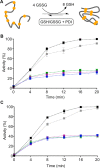
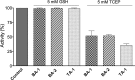
The renewed use of arsenicals as chemotherapeutics has rekindled interest in the biochemistry of As(III) species. In this work, simple bis- and tris-arsenical derivatives were synthesized with the aim of exploiting the chelate effect in the inhibition of thiol-disulfide oxidoreductases (here, Quiescin sulfhydryl oxidase, QSOX, and protein disulfide isomerase, PDI) that utilize two or more CxxC motifs in the catalysis of oxidative protein folding. Coupling 4-aminophenylarsenoxide (APAO) to acid chloride or anhydride derivatives yielded two bis-arsenical prototypes, BA-1 and BA-2, and a tris-arsenical, TA-1. Unlike the monoarsenical, APAO, these new reagents proved to be strong inhibitors of oxidative protein folding in the presence of a realistic intracellular concentration of competing monothiol (here, 5 mM reduced glutathione, GSH). However, this inhibition does not reflect direct inactivation of QSOX or PDI, but avid binding of MVAs to the reduced unfolded protein substrates themselves. Titrations of reduced riboflavin-binding protein with MVAs show that all 18 protein -SH groups can be captured by these arsenicals. With reduced RNase, addition of substoichiometric levels of MVAs is accompanied by the formation of Congo Red- and Thioflavin T-positive fibrillar aggregates. Even with Kd values of ∼50 nM, MVAs are ineffective inhibitors of PDI in the presence of millimolar levels of competing GSH. These results underscore the difficulties of designing effective and specific arsenical inhibitors for folded enzymes and proteins. Some of the cellular effects of arsenicals likely reflect their propensity to associate very tightly and nonspecifically to conformationally mobile cysteine-rich regions of proteins, thereby interfering with folding and/or function.
Figures

Similar articles
-
Quiescin sulfhydryl oxidase from Trypanosoma brucei: catalytic activity and mechanism of a QSOX family member with a single thioredoxin domain.Biochemistry. 2010 Mar 9;49(9):2075-85. doi: 10.1021/bi902222s. Biochemistry. 2010. PMID: 20121244 Free PMC article.
-
Oxidative protein folding: from thiol-disulfide exchange reactions to the redox poise of the endoplasmic reticulum.Free Radic Biol Med. 2015 Mar;80:171-82. doi: 10.1016/j.freeradbiomed.2014.07.037. Epub 2014 Aug 1. Free Radic Biol Med. 2015. PMID: 25091901 Free PMC article. Review.
-
Challenges in the evaluation of thiol-reactive inhibitors of human protein disulfide Isomerase.Free Radic Biol Med. 2017 Jul;108:741-749. doi: 10.1016/j.freeradbiomed.2017.04.367. Epub 2017 Apr 30. Free Radic Biol Med. 2017. PMID: 28465261 Free PMC article.
-
Arsenic(III) species inhibit oxidative protein folding in vitro.Biochemistry. 2009 Jan 20;48(2):424-32. doi: 10.1021/bi801988x. Biochemistry. 2009. PMID: 19102631 Free PMC article.
-
Oxidative protein folding and the Quiescin-sulfhydryl oxidase family of flavoproteins.Antioxid Redox Signal. 2010 Oct;13(8):1217-30. doi: 10.1089/ars.2010.3098. Antioxid Redox Signal. 2010. PMID: 20136510 Free PMC article. Review.
Cited by
-
Gaussia princeps luciferase: a bioluminescent substrate for oxidative protein folding.Protein Sci. 2018 Aug;27(8):1509-1517. doi: 10.1002/pro.3433. Epub 2018 Jul 18. Protein Sci. 2018. PMID: 29696739 Free PMC article.
-
Arsenic induced redox imbalance triggers the unfolded protein response in the liver of zebrafish.Toxicol Appl Pharmacol. 2020 Dec 15;409:115307. doi: 10.1016/j.taap.2020.115307. Epub 2020 Nov 2. Toxicol Appl Pharmacol. 2020. PMID: 33147493 Free PMC article.
-
Inorganic arsenic causes fatty liver and interacts with ethanol to cause alcoholic liver disease in zebrafish.Dis Model Mech. 2018 Feb 26;11(2):dmm031575. doi: 10.1242/dmm.031575. Dis Model Mech. 2018. PMID: 29361514 Free PMC article.
-
Direct binding of arsenicals to nuclear transport factors disrupts nucleocytoplasmic transport.bioRxiv [Preprint]. 2025 May 21:2025.01.13.632748. doi: 10.1101/2025.01.13.632748. bioRxiv. 2025. Update in: J Cell Sci. 2025 Aug 15;138(16):jcs263889. doi: 10.1242/jcs.263889. PMID: 39868121 Free PMC article. Updated. Preprint.
-
Mia40 is a facile oxidant of unfolded reduced proteins but shows minimal isomerase activity.Arch Biochem Biophys. 2015 Aug 1;579:1-7. doi: 10.1016/j.abb.2015.05.005. Epub 2015 May 23. Arch Biochem Biophys. 2015. PMID: 26014136 Free PMC article.
References
-
- Wang Z. Y.; Chen Z. (2008) Acute promyelocytic leukemia: from highly fatal to highly curable. Blood 111, 2505–2515. - PubMed
-
- Wu J.; Henderson C.; Feun L.; Van Veldhuizen P.; Gold P.; Zheng H.; Ryan T.; Blaszkowsky L. S.; Chen H.; Costa M.; Rosenzweig B.; Nierodzik M.; Hochster H.; Muggia F.; Abbadessa G.; Lewis J.; Zhu A. X. (2010) Phase II study of darinaparsin in patients with advanced hepatocellular carcinoma. Invest. New Drugs 28, 670–676. - PubMed
-
- Nidhubhghaill O. M.; Sadler P. J. (1991) The structure and reactivity of arsenic compounds—biological activity and drug design. Struct. Bonding (Berlin, Ger.) 78, 129–190.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

