STIM1 positively regulates the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor in bovine aortic endothelial cells
- PMID: 25506690
- PMCID: PMC4266619
- DOI: 10.1371/journal.pone.0114718
STIM1 positively regulates the Ca2+ release activity of the inositol 1,4,5-trisphosphate receptor in bovine aortic endothelial cells
Abstract
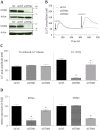
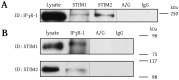
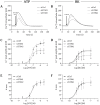
The endothelium is actively involved in many functions of the cardiovascular system, such as the modulation of arterial pressure and the maintenance of blood flow. These functions require a great versatility of the intracellular Ca2+ signaling that resides in the fact that different signals can be encoded by varying the frequency and the amplitude of the Ca2+ response. Cells use both extracellular and intracellular Ca2+ pools to modulate the intracellular Ca2+ concentration. In non-excitable cells, the inositol 1,4,5-trisphosphate receptor (IP3R), located on the endoplasmic reticulum (ER), is responsible for the release of Ca2+ from the intracellular store. The proteins STIM1 and STIM2 are also located on the ER and they are involved in the activation of a store-operated Ca2+ entry (SOCE). Due to their Ca2+ sensor property and their close proximity with IP3Rs on the ER, STIMs could modulate the activity of IP3R. In this study, we showed that STIM1 and STIM2 are expressed in bovine aortic endothelial cells and they both interact with IP3R. While STIM2 appears to play a minor role, STIM1 plays an important role in the regulation of agonist-induced Ca2+ mobilization in BAECs by a positive effect on both the SOCE and the IP3R-dependent Ca2+ release.
Conflict of interest statement
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

