Direct conversion of mouse and human fibroblasts to functional melanocytes by defined factors
- PMID: 25510211
- PMCID: PMC4335710
- DOI: 10.1038/ncomms6807
Direct conversion of mouse and human fibroblasts to functional melanocytes by defined factors
Abstract
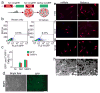
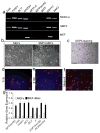
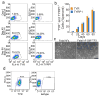
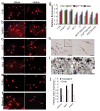
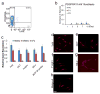
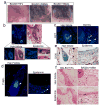
Direct reprogramming provides a fundamentally new approach for the generation of patient-specific cells. Here, by screening a pool of candidate transcription factors, we identify that a combination of the three factors, MITF, SOX10 and PAX3, directly converts mouse and human fibroblasts to functional melanocytes. Induced melanocytes (iMels) activate melanocyte-specific networks, express components of pigment production and delivery system and produce melanosomes. Human iMels properly integrate into the dermal-epidermal junction and produce and deliver melanin pigment to surrounding keratinocytes in a 3D organotypic skin reconstruct. Human iMels generate pigmented epidermis and hair follicles in skin reconstitution assays in vivo. The generation of iMels has important implications for studies of melanocyte lineage commitment, pigmentation disorders and cell replacement therapies.
Figures


References
-
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev. 2004;84:1155–1228. - PubMed
-
- Alikhan A, Felsten LM, Daly M, Petronic-Rosic V. Vitiligo: a comprehensive overview Part I. Introduction, epidemiology, quality of life, diagnosis, differential diagnosis, associations, histopathology, etiology, and work-up. J Am Acad Dermatol. 2011;65:473–491. - PubMed
-
- Hann SK, Im S, Bong HW, Park YK. Treatment of stable vitiligo with autologous epidermal grafting and PUVA. J Am Acad Dermatol. 1995;32:943–948. - PubMed
-
- Caiazzo M, et al. Direct generation of functional dopaminergic neurons from mouse and human fibroblasts. Nature. 2011;476:224–227. - PubMed
-
- Huang P, et al. Induction of functional hepatocyte-like cells from mouse fibroblasts by defined factors. Nature. 2011;475:386–389. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

